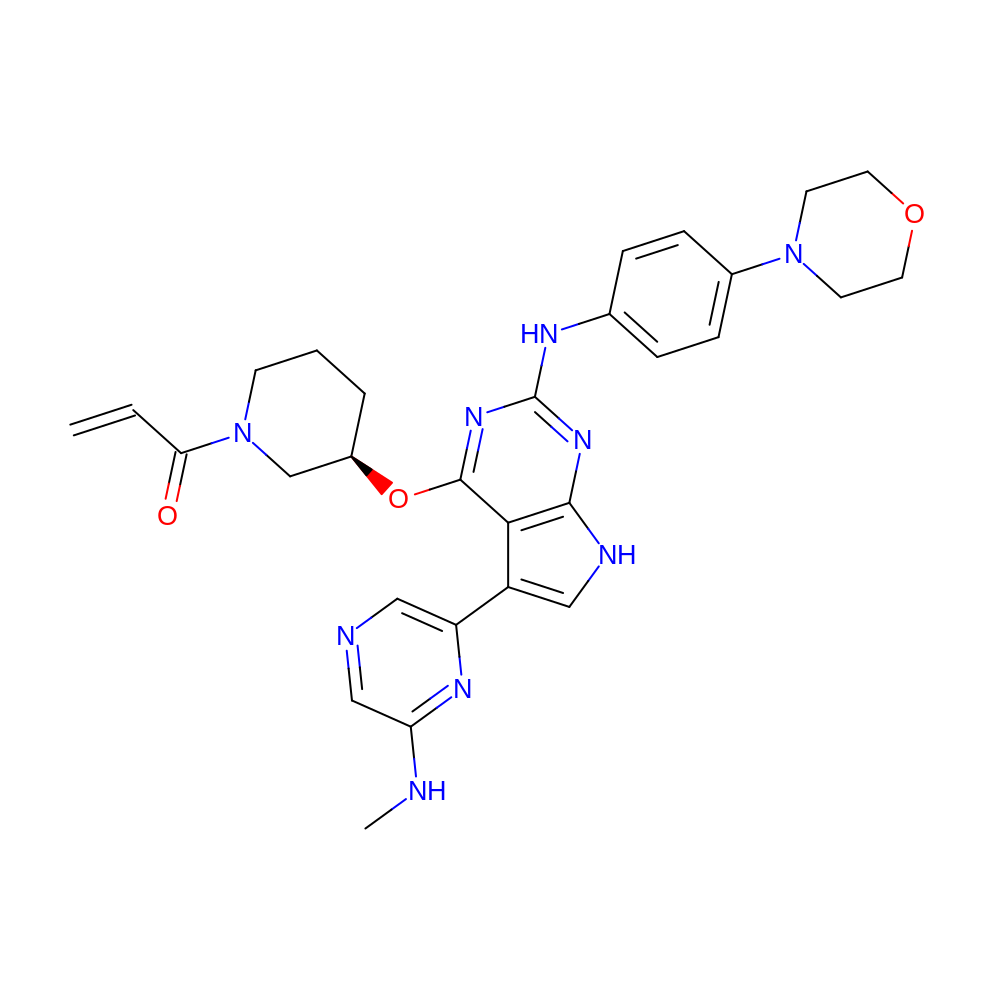
1-[(3R)-3-[[5-[6-(methylamino)pyrazin-2-yl]-2-(4-morpholinoanilino)-7H-pyrrolo[2,3-d]pyrimidin-4-yl]oxy]-1-piperidyl]prop-2-en-1-one
Inhibitor information
- CovInDB Inhibitor
- CI005348
- Name
- 1-[(3R)-3-[[5-[6-(methylamino)pyrazin-2-yl]-2-(4-morpholinoanilino)-7H-pyrrolo[2,3-d]pyrimidin-4-yl]oxy]-1-piperidyl]prop-2-en-1-one
- Molecular Formula
- C29H33N9O3
- Molecular Weight
- 555.2706359 g/mol
- Structure
-
- IUPAC Name
- 1-[(3R)-3-[[5-[6-(methylamino)pyrazin-2-yl]-2-(4-morpholinoanilino)-7H-pyrrolo[2,3-d]pyrimidin-4-yl]oxy]-1-piperidyl]prop-2-en-1-one
- InChI
- InChI=1S/C29H33N9O3/c1-3-25(39)38-10-4-5-21(18-38)41-28-26-22(23-16-31-17-24(30-2)34-23)15-32-27(26)35-29(36-28)33-19-6-8-20(9-7-19)37-11-13-40-14-12-37/h3,6-9,15-17,21H,1,4-5,10-14,18H2,2H3,(H,30,34)(H2,32,33,35,36)/t21-/m1/s1
- InChI Key
- XTRQLWDUMOTTDA-OAQYLSRUSA-N
- Canonical SMILES
- C=CC(=O)N1CCC[C@@H](Oc2nc(Nc3ccc(N4CCOCC4)cc3)nc3[nH]cc(-c4cncc(NC)n4)c23)C1
- Cocrystal structures
- No cocrystal structures found for this inhibitor.
Calculated Properties
- Molecular Weight
-
555.2706359 g/mol
Computed by RDKit
- logP
-
4.761
Computed by ALOGPS
- logS
-
-5.88
Computed by ALOGPS
- Heavy Atom Count
-
41
Computed by RDKit
- Ring Count
-
6
Computed by RDKit
- Hydrogen Bond Acceptor Count
-
10
Computed by RDKit
- Hydrogen Bond Donor Count
-
3
Computed by RDKit
- Rotatable Bond Count
-
8
Computed by RDKit
- Topological Polar Surface Area
-
133.42 Å2
Computed by RDKit
3D Structure
targets
| Name | ID | Warhead | Reaction Mechanism | Target Site | Activity Type | Relation | Value | Unit | Experiment Method | Assay | Reference |
|---|
selectivity
| Target | Activity Type | Relation | Value | Unit | Assay | Reference |
|---|
Similar compounds in Virtual Screening library
No similar compounds in the virtual screening library found for this inhibitor.
Similar Natural compounds
No similar natural compounds found for this inhibitor.