Introduction
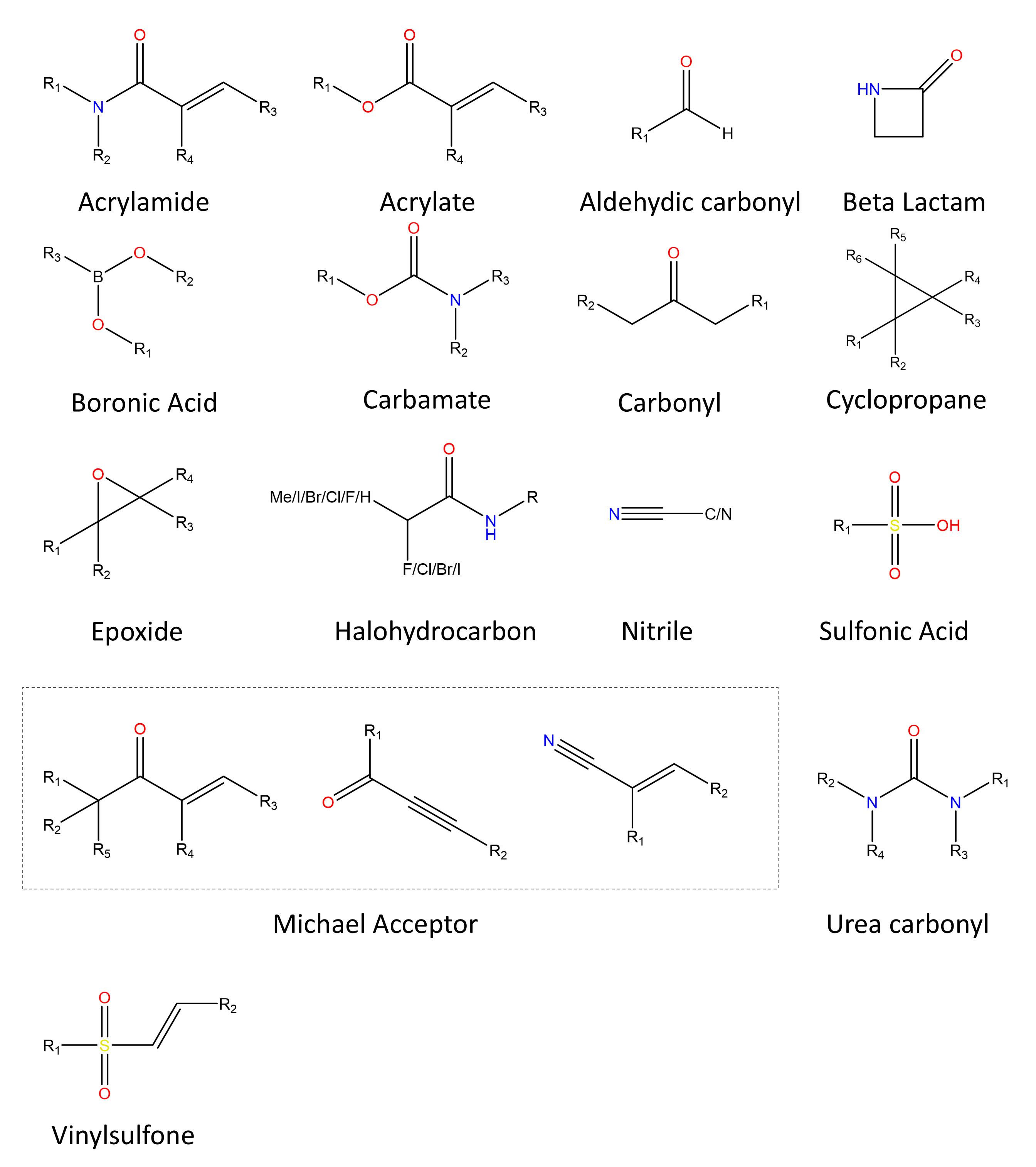
✔ Covalent inhibitors are usually small molecules that bind to enzymes through covalent bonds and inactive them temporarily or permanently. Compared with noncovalent inhibitors, covalent inhibitors have the advantages of longer residence time, higher potency, and decreased drug resistance. A covalent inhibitor usually contains two parts: a noncovalent fragment and an electrophilic covalent reactive group (also called warhead). The latter, which is essential for a covalent inhibitor, usually determines its reactivity, thus influencing the potency and toxicity of the inhibitor.
✔ The covalent inhibitor database (CovalentInDB) is a web-accessible open resource for covalent inhibitors and related targets. You can not only get the basic information of the inhibitors, targets and bioactivity data, but also the essential information about the covalent reaction, such as the warhead of the inhibitor, the covalent reaction mechanism, the reaction site and the covalent-mechanism experimental verification method. CovalentInDB 2.0 contains several key types of data: 1) structural and biological activity data of covalent inhibitors, including covalent inhibition of target, activity in cell lines or organism, selectivity indicated by inhibitory effects on other targets, ADMET properties, and intrinsic reactivity (often measured by the reaction of inhibitors with glutathione); 2) cocrystal structures of covalent inhibitor-target complexes; 3) ligandability information for cysteines predicted by DeepCoSI; 4) a natural product library with covalent inhibition potential; and 5) a covalent virtual screening library designed for covalent drug discovery.
Tutorial
✔ Home Page:
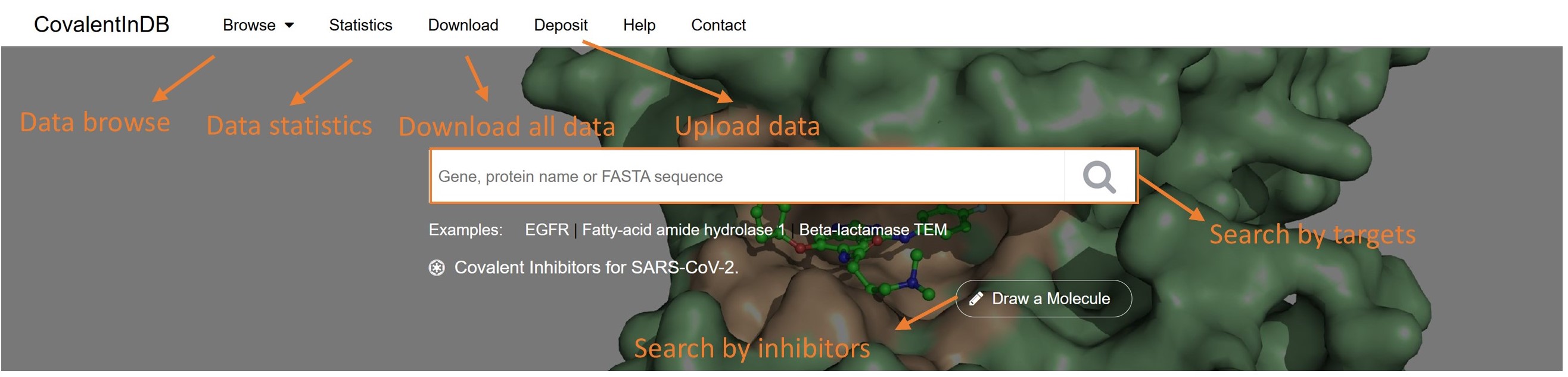
Searching for a Target
To search for a target, simply type in its gene, protein name or UniProt protein identifier and click the button 'Search'. You can also use the sequence-based search engine by pasting a FASTA format sequence in the search box.
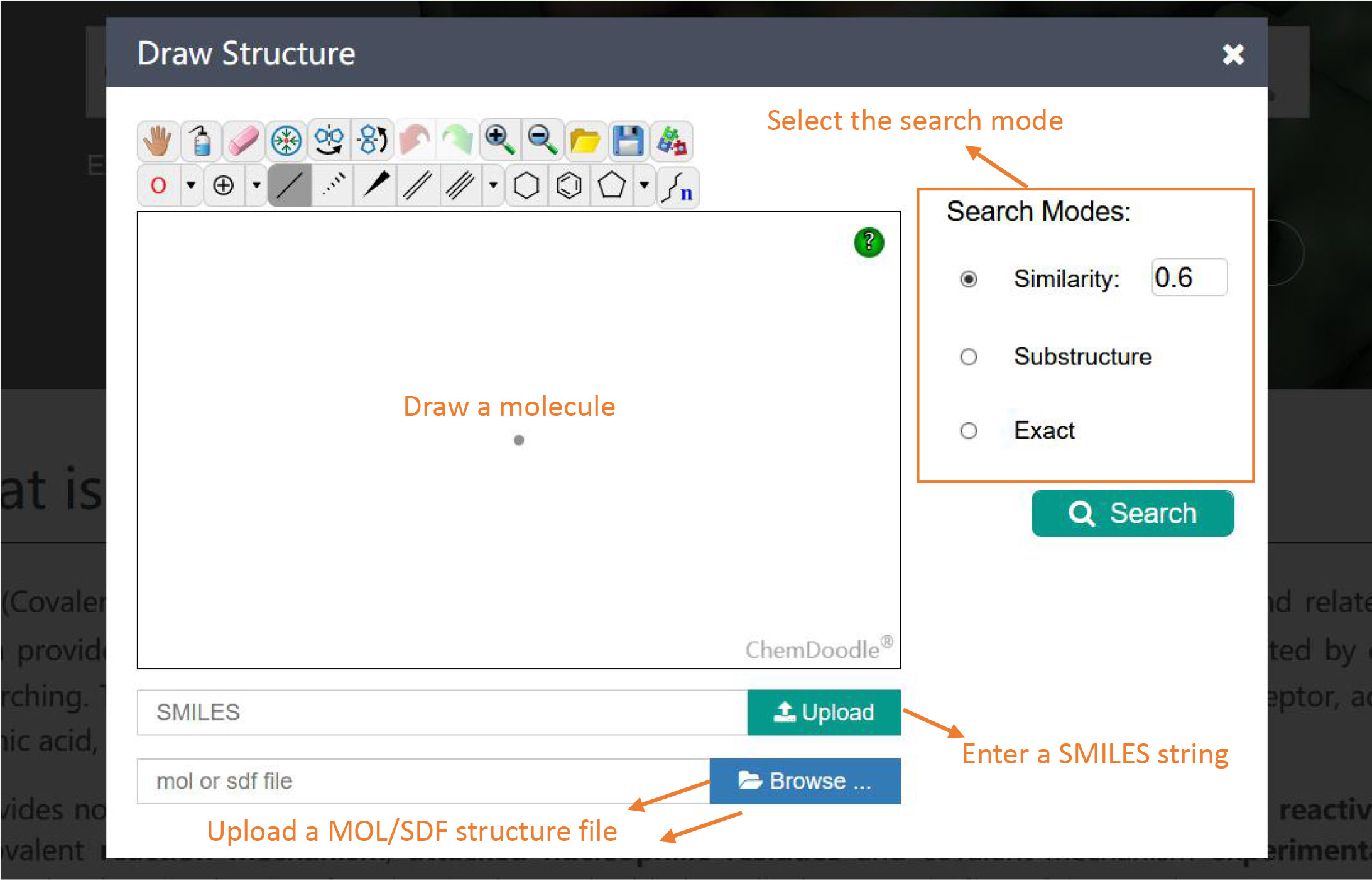
Searching for an Inhibitor
To search for an inhibitor, click the button 'Draw a Molecule'. You can input a SMILES string, upload a MOL/SDF structure file or sketch a molecule within the ChemDoodle editor. After the query molecule has been imported, one of the three searching options (i.e., similarity, substructure or exact) can be chosen. Please note that you can only draw a single molecule each time. And if you want to draw a second molecule, please refresh the page.
Navigation bar
Browse: Retrieve the information in a more convenient way.
Statistics: Click here, you will get the statistics of the data in CovalentInDB.
Download: If you want to download the data in CovalentInDB, please click here. The data are sorted by the Targets, Warheads, Reaction Types.
Deposit: Click here, you can contribute your own data to the CovalentInDB.
Help: Click here and you will jump to this page for help.
✔ Target Page:
This page contains three sections: Target information, Cocrystal structure and Covalent inhibitors.
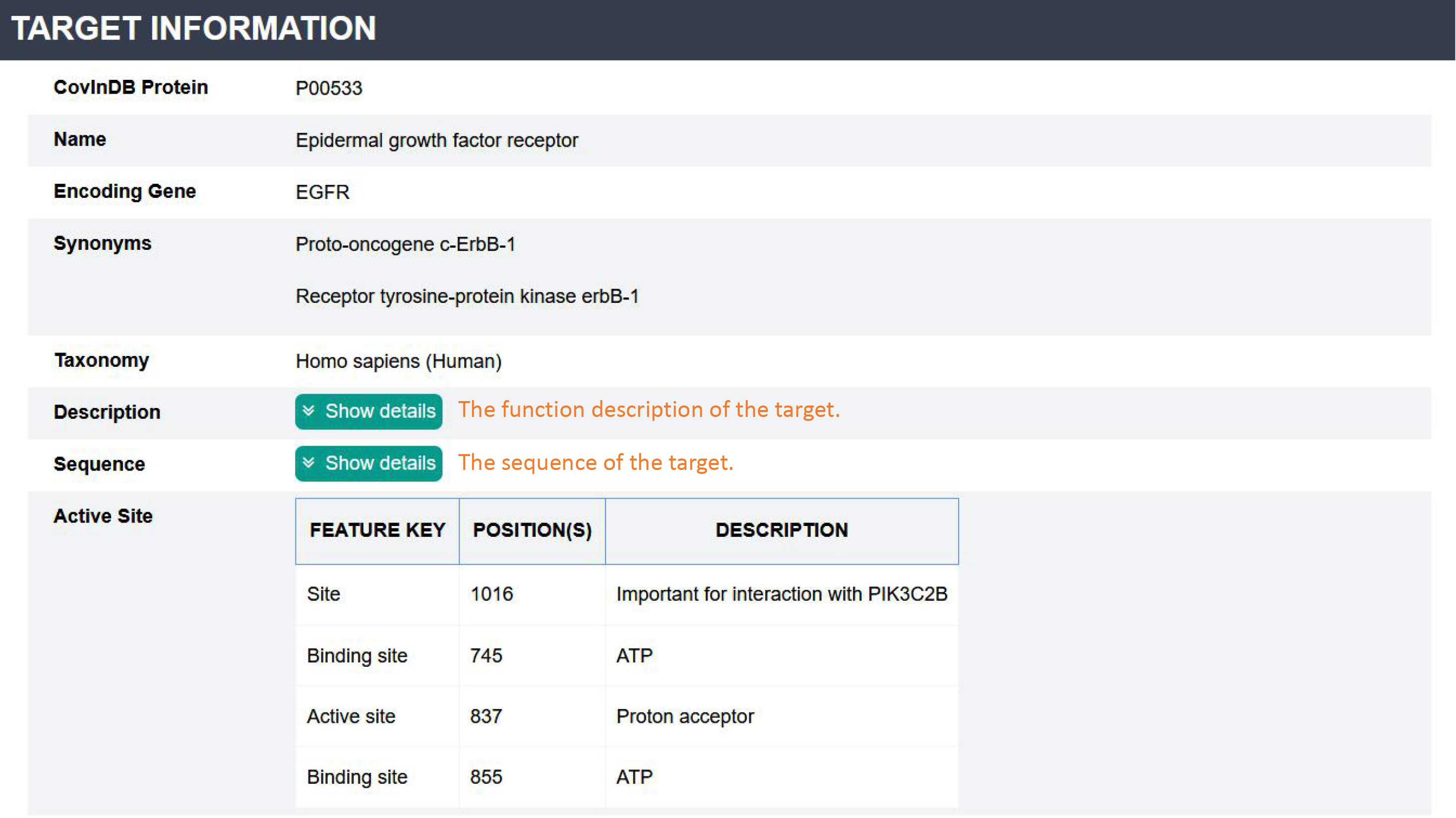
Target information: This section contains the basic information of each target, including UniProt ID, Name, Gene, Synonyms, Taxonomy, Function description, Sequence and Active site.
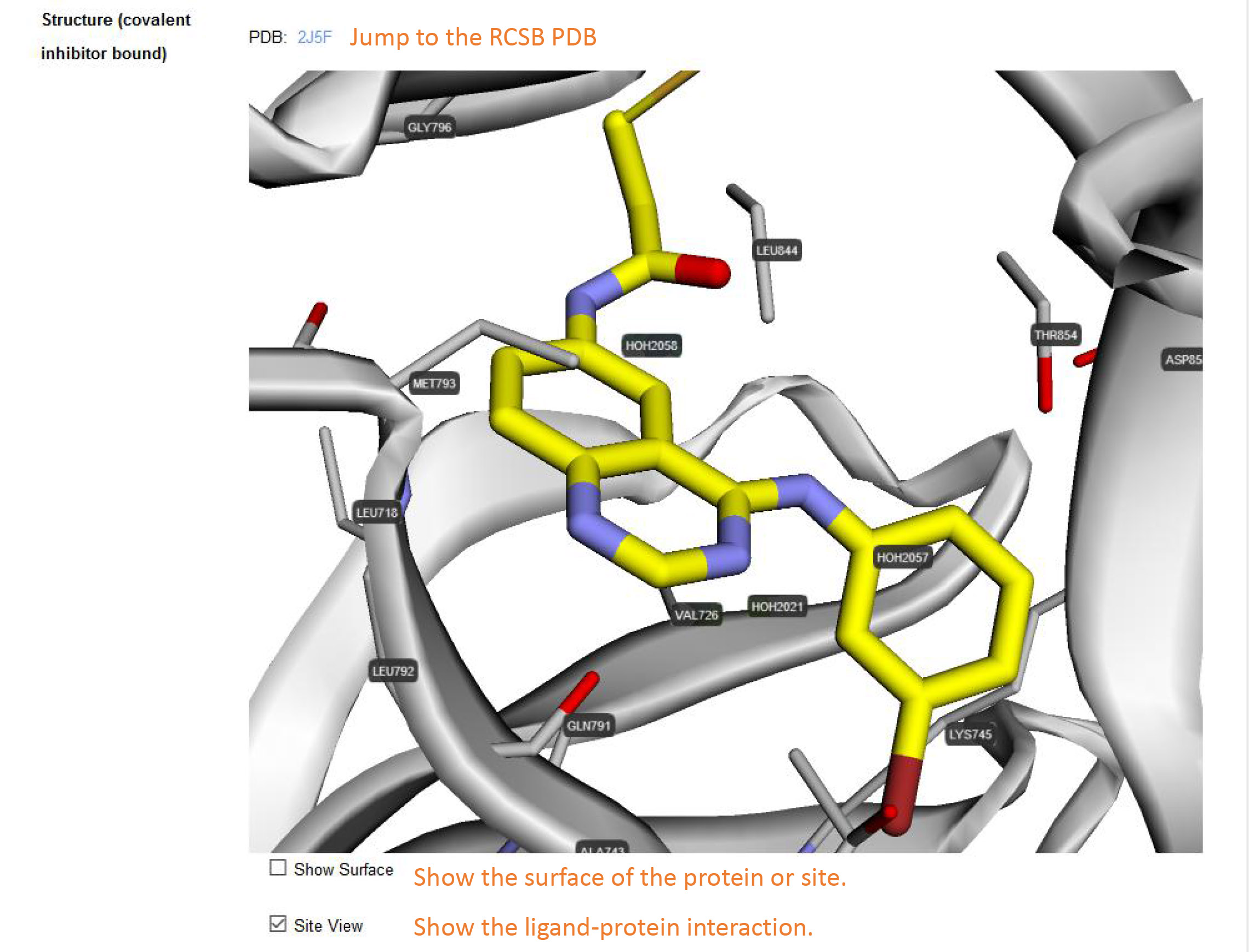
Cocrystal structure: This section displays the cocrystal structure of the target bound to a covalent inhibitor. The PDBs are downloaded from the Protein Data Bank (PDB). Not all targets in CovalentInDB have the cocrystal structures in complex with covalent inhibitors. Please check 'Site View' to show the ligand-target interaction. The amino acids within 5 Å around the inhibitor are shown as stick. A semitransparent surface will be added to the whole structure or the site if you check the 'Show Surface'.
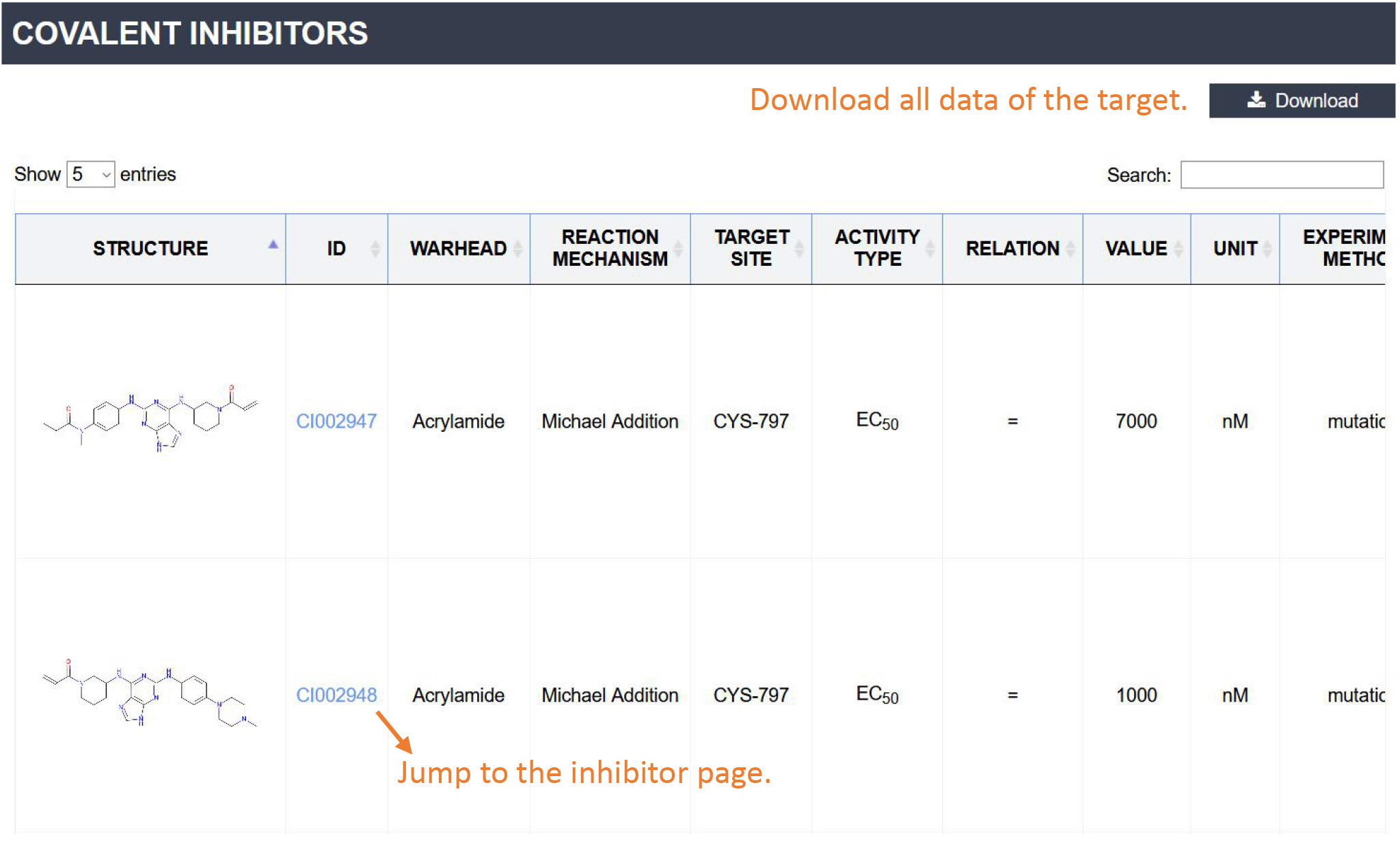
Covalent inhibitors: This section contains all the covalent inhibitors of the target. For each inhibitor, the table displays the Structure, ID, Warhead, Reaction mechanism, Site, Bioactivity, Experimental verification method, Bioassay and Reference. You can click the 'Structure' or 'ID' to jump to the Inhibitor Page. To download the data of this target, please click the "Download" button.
✔ Inhibitor Page:
This page contains five sections: Inhibitor representations, Calculated properties, 3D structure ,Related targets and Similar compounds in the natural product and covalent virtual screening libraries.
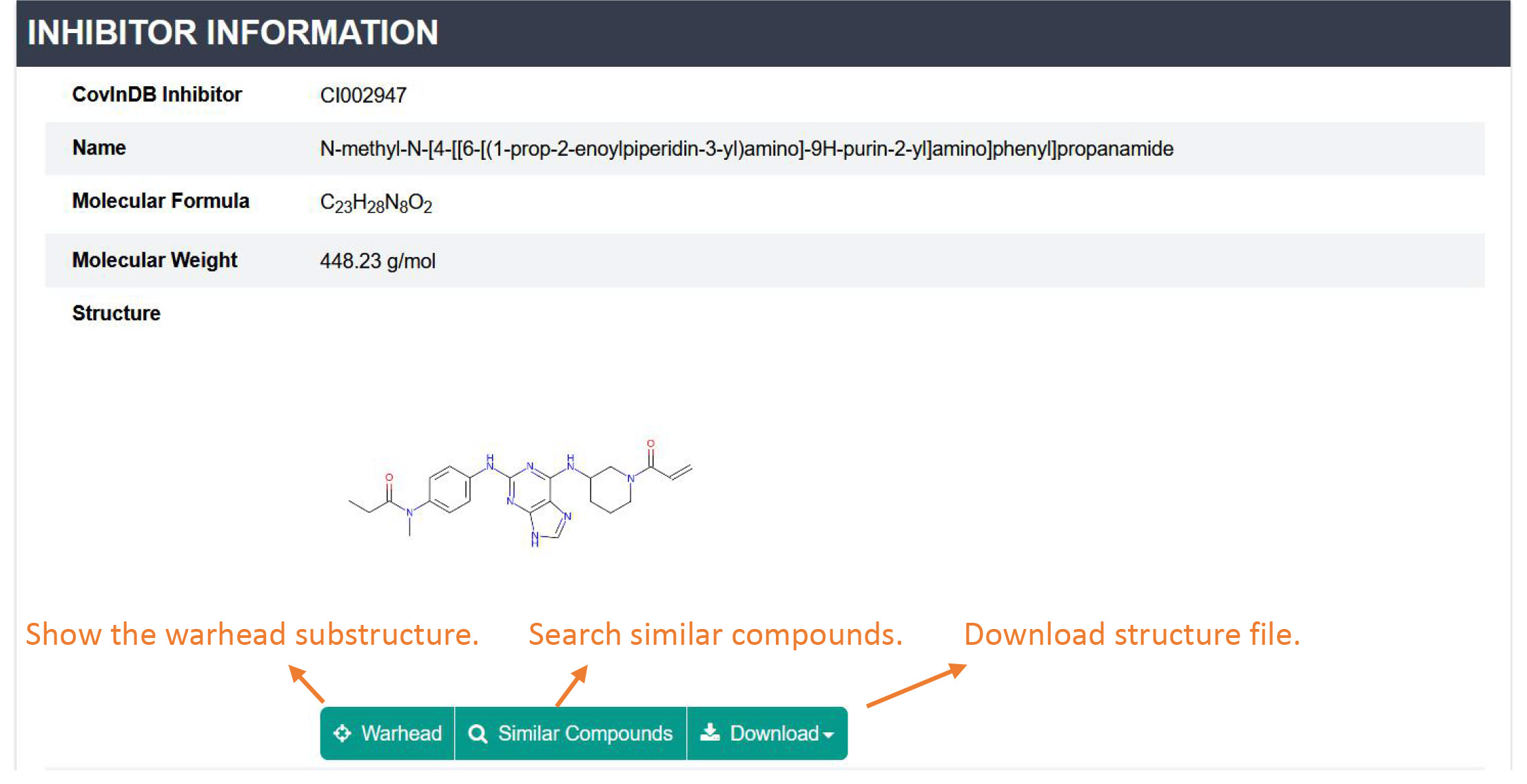
Inhibitor representations: This section contains the basic information of the inhibitor, including ID, Name, Molecular formula, Molecular weight, Structure, IUPAC name, InChI, InChI key and SMILES. Click the button 'Warhead' and you will jump to the 3D Structure section. CovalentInDB allows users to retrieve the similar inhibitors of a compound of interest and check their related targets. To download the structure file, please click the 'Download' button.
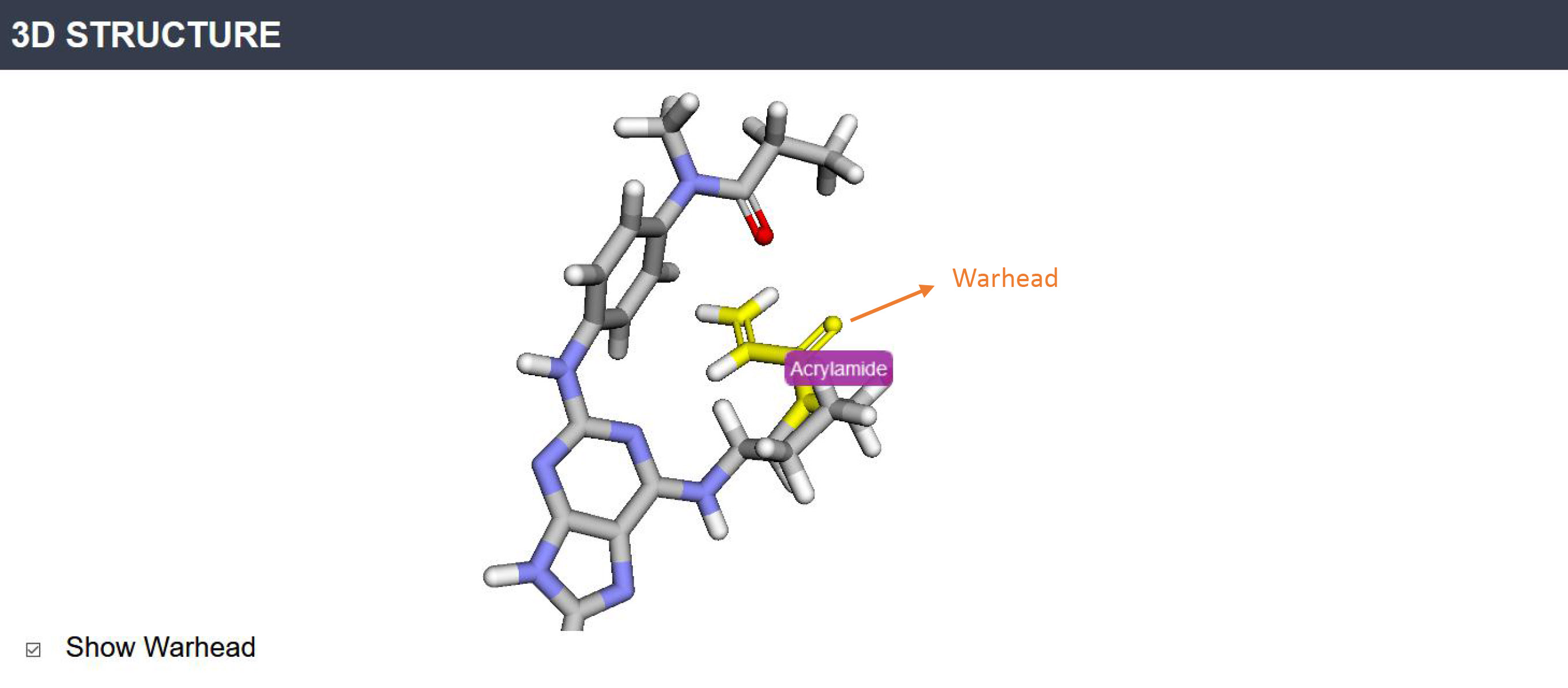
3D structure: The warhead substructure will be highlighted with a label by pressing the 'Show Warhead' button.
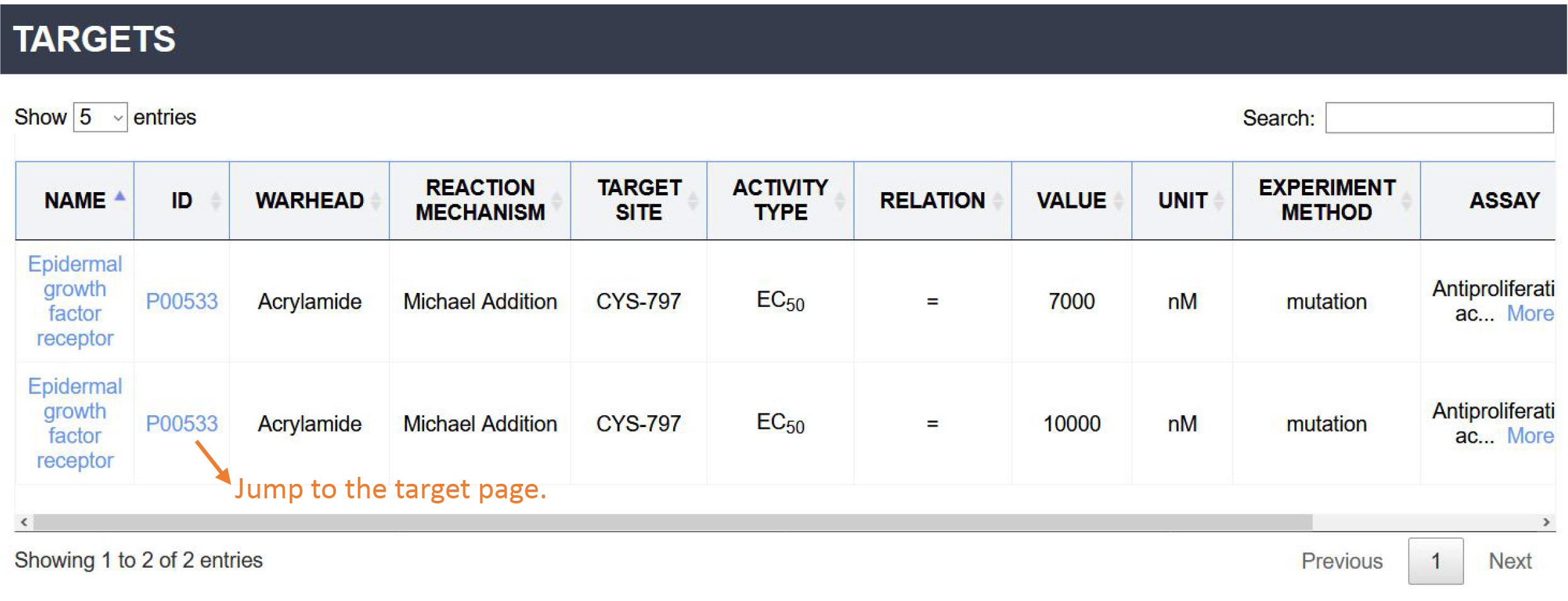
Related targets: This section contains all the targets that relate to the inhibitor. For each target, the table displays the Name, ID, Warhead, Reaction mechanism, Site, Bioactivity, Experimental verification method, Bioassay and Reference. You can click the 'Name' or 'ID' to jump to the Target Page.
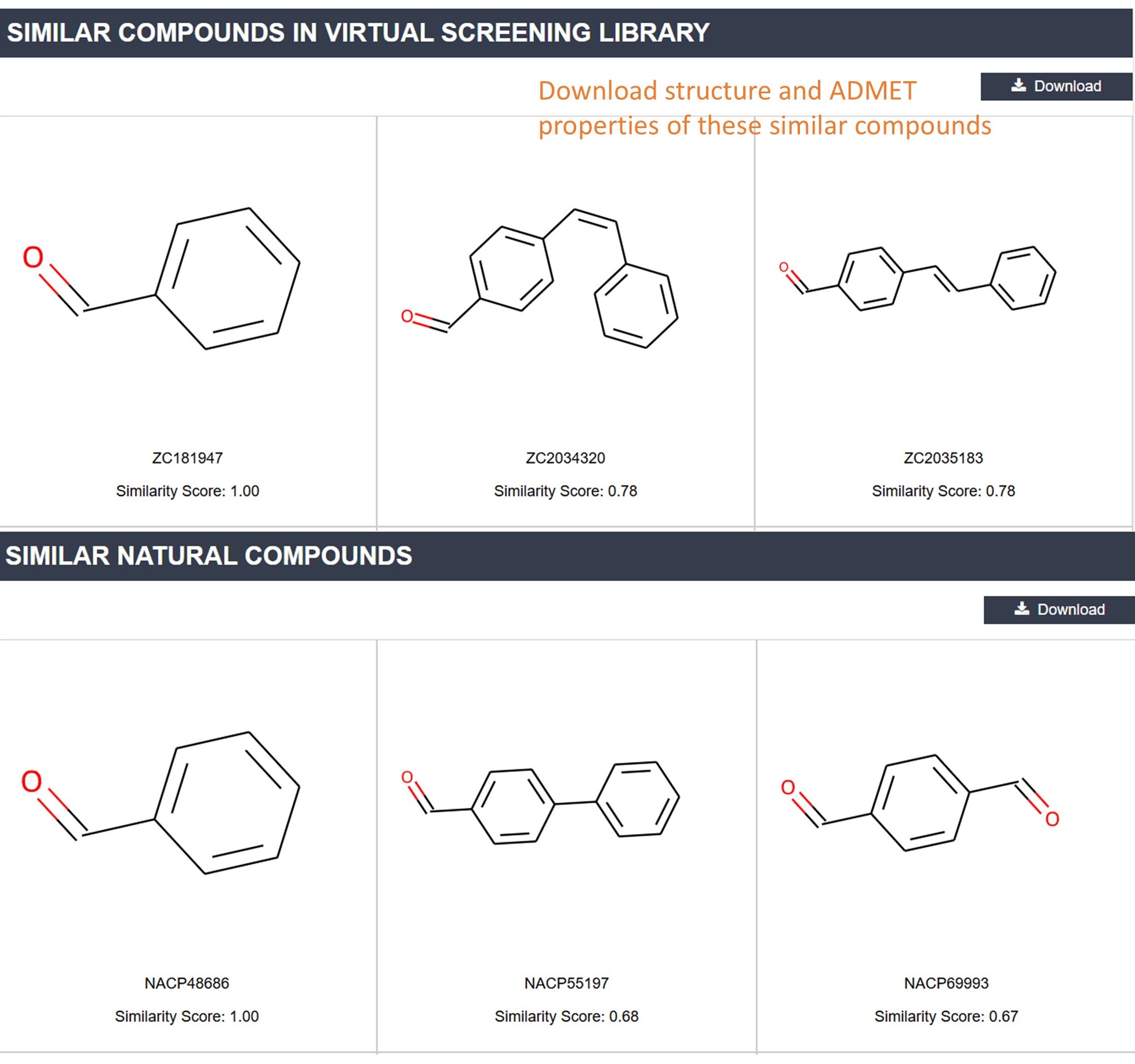
Similar Compounds in libraries: This section displays natural products or compounds from the covalent virtual screening library that are similar to this inhibitor. You can download the structure and ADMET properties of these compounds.
✔ Data Browse:
CovalentInDB provides four data browsing functions to retrieve the information of interest more conveniently: 'Warheads browse', 'Reaction mechanism browse', 'Binding sites browse' and 'Covalent drugs browse'.
For example, 'Warheads browse' allows users to display the list of all covalent inhibitors possessing a specific warhead. For each warhead, a molecular visualization program is embedded to highlight the warhead structure and a diagram is plotted to show the structural transformation during covalent reaction.
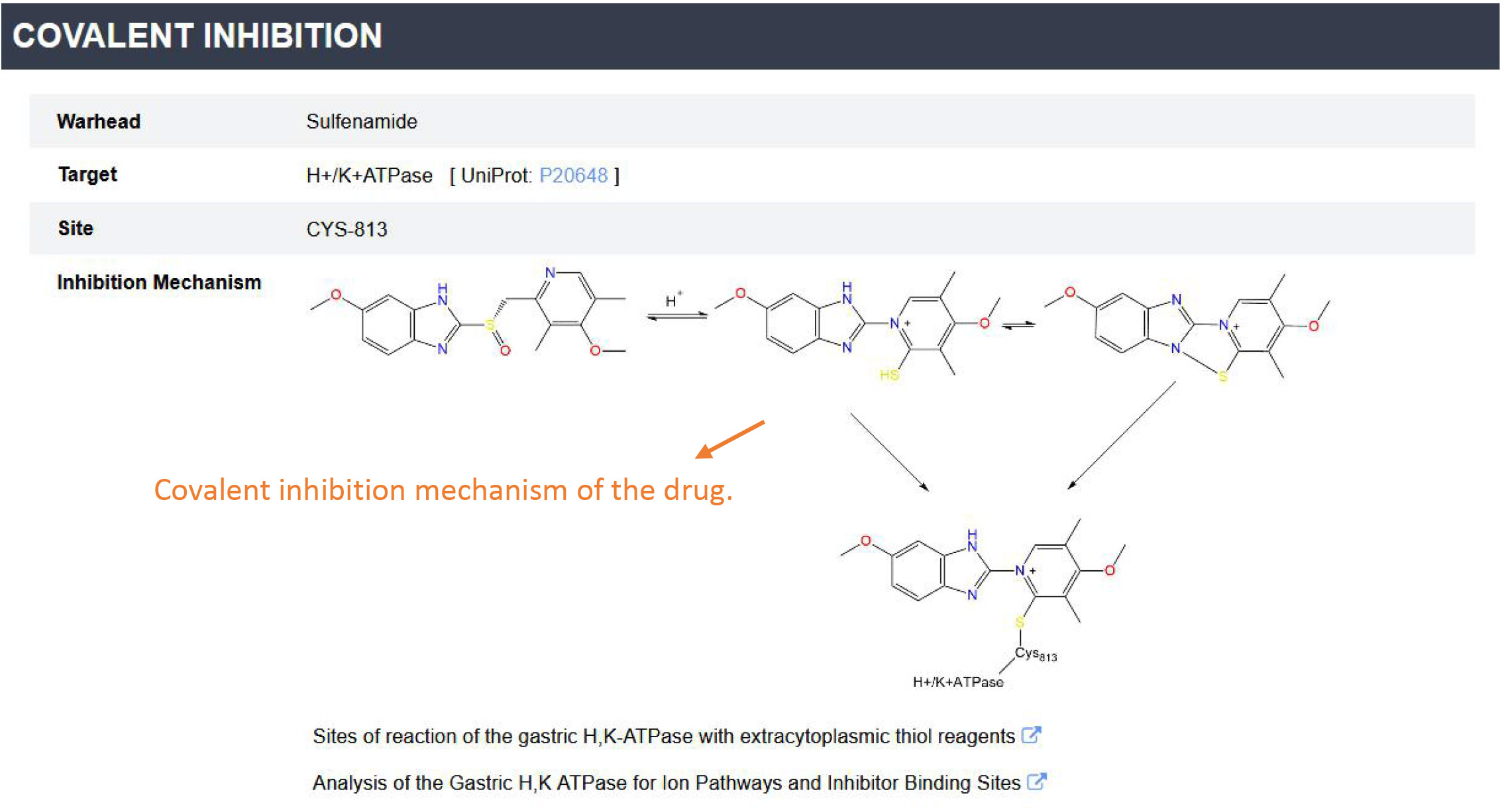
'Covalent drugs browse' provides a convenient way to summarize the information for each covalent drug. By selecting a drug of interest and clicking the 'Submit' button, the detailed covalent inhibition information for this drug will be displayed.
✔ Covalent-binding Complexes:
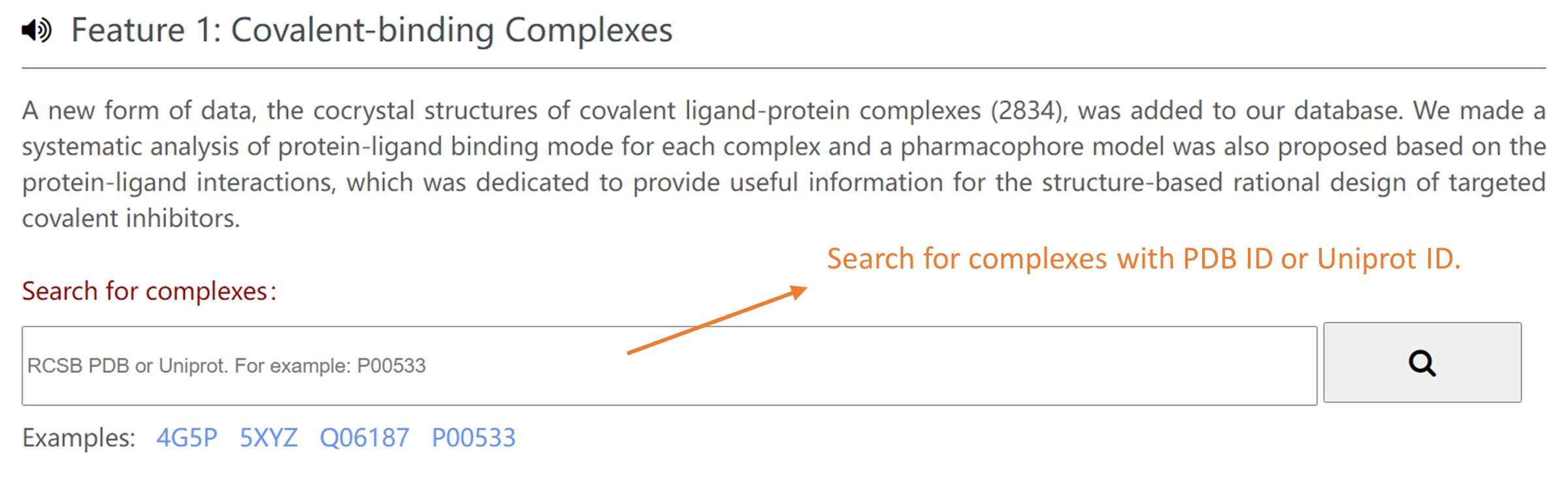
You can get the covalent ligand-protein cocrystal structures with PDB ID or Uniprot ID in Home Page. You can also browse the structures based on their warheads/reaction mechanism/binding sites in Browse Page.
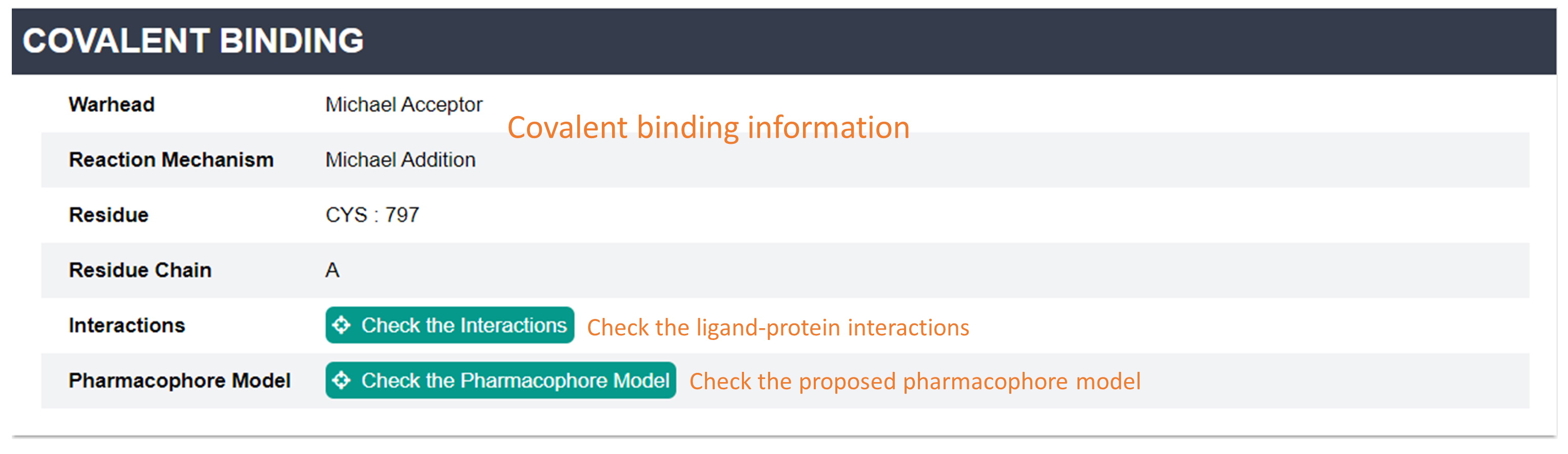
Structure Information: This page contains three sections: basic information for the PDB structure, ligand structure (before covalent reaction) and covalent binding information. You can check the ligand-protein interactions and pharmacophore model by clicking the buttons.
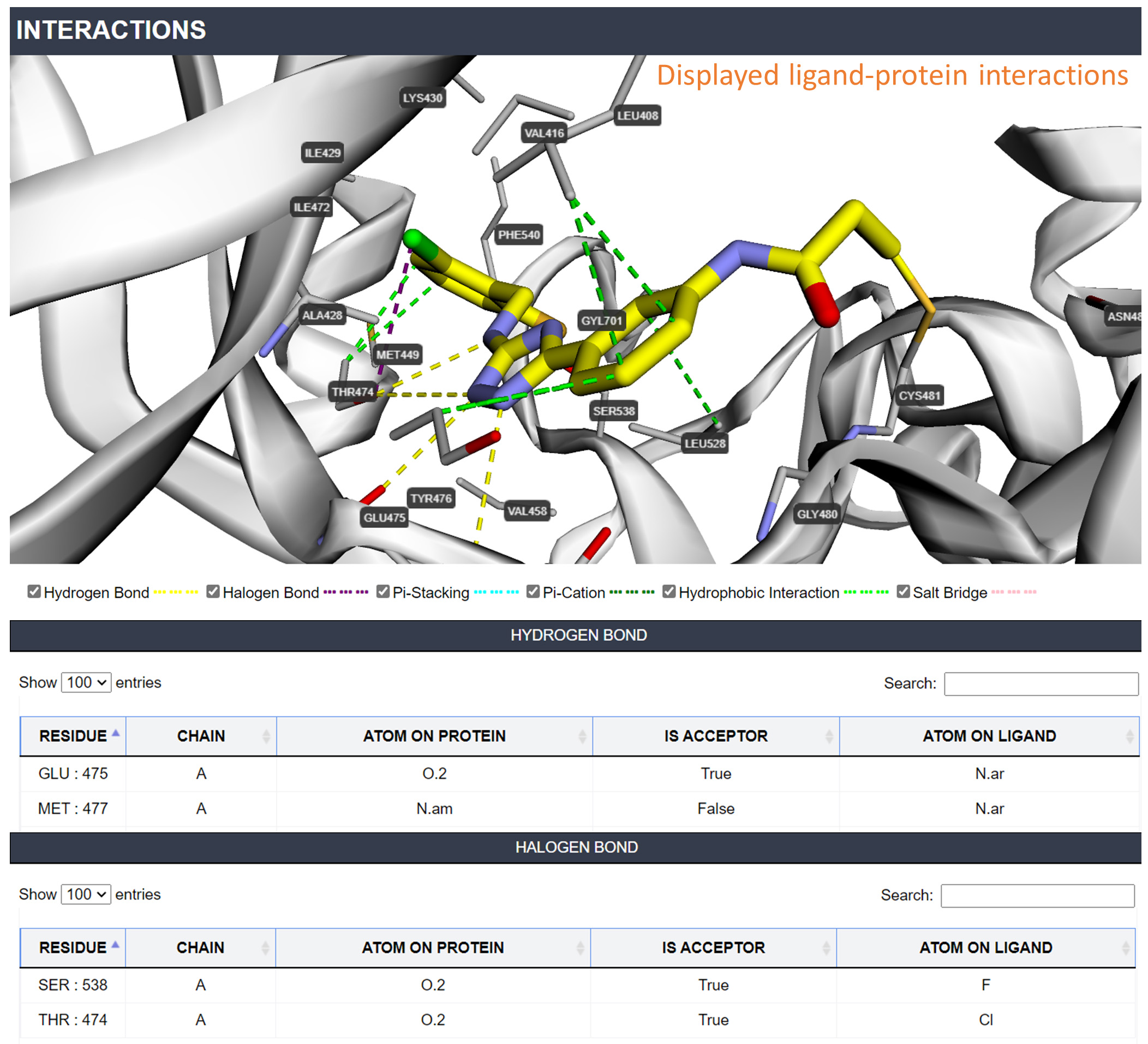
Interactions: You can check the binding mode of each ligand-protein complex in this page. Six types of interactions are displayed including Hydrogen Bond/Halogen Bond/Pi-Stacking/Pi-Cation/Hydrophobic Interaction/Salt Bridge.

Pharmacophore Model: In this page, You can check the pharmacophore model which was proposed based on the interactions between ligand and receptor.
✔ Profiled Covalent Binding Sites
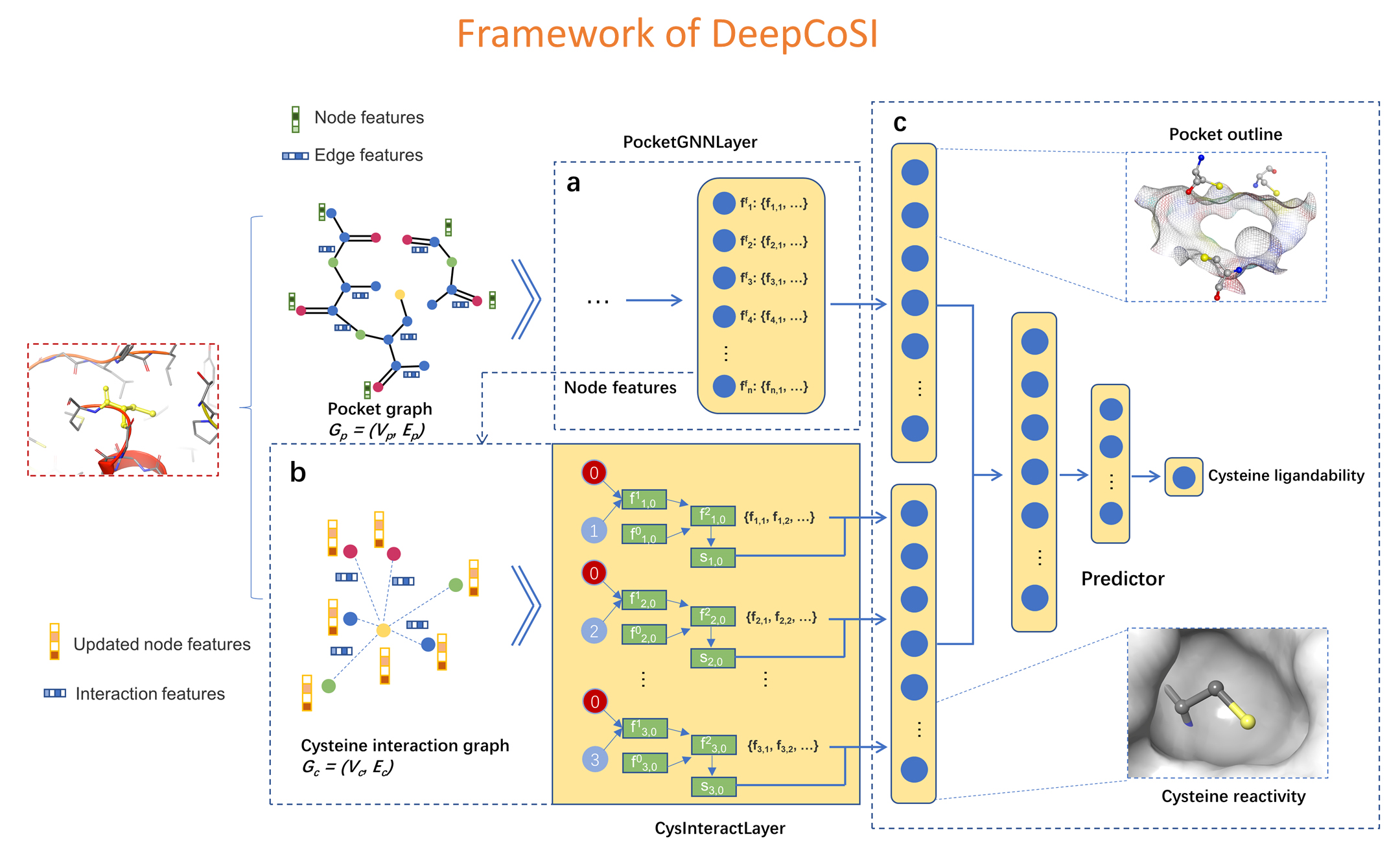
DeepCoSI: DeepDoSI is a structure-based deep graph learning model to identify covalent binding sites. By integrating the characterization of the binding pocket and the interactions between each cysteine and the surrounding environment, DeepCoSI achieves state-of-the-art predictive performances (AUPRC = 0.82). The validation on two external test sets which mimics the real application scenarios shows that DeepCoSI has strong ability to distinguish ligandable cysteines from the others. In order to expand the discovery of covalent ligands to the proteome-level, we profiled the entire protein structures in RCSB Protein Data Bank (PDB) with DeepCoSI to evaluate the covalent-ligandability of each cysteine.
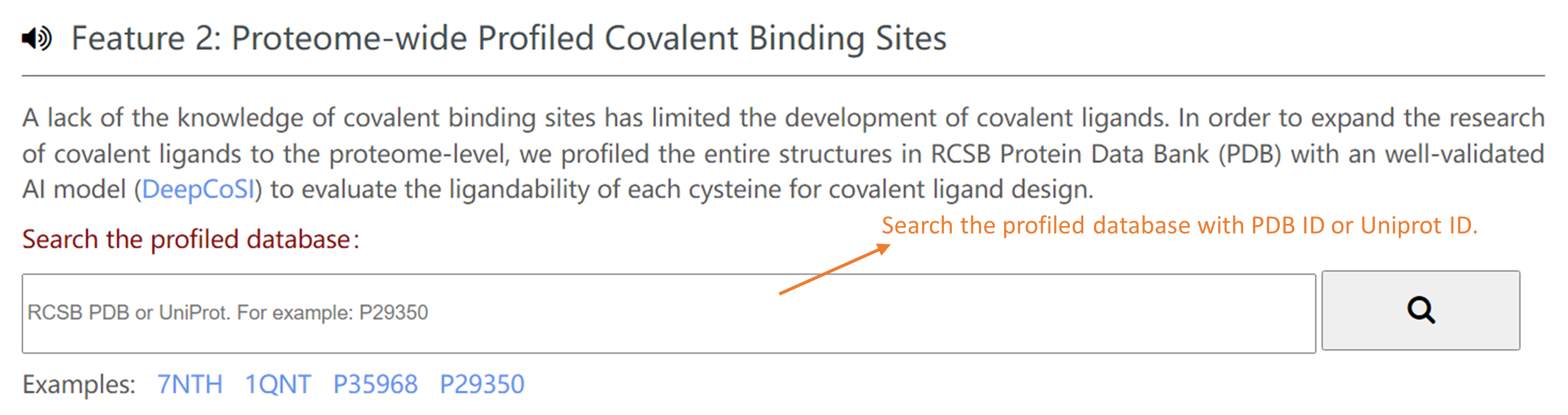
Profiled database: In order to expand the discovery of covalent ligands to the proteome-level,we profiled the entire protein structures in RCSB Protein Data Bank (PDB) with DeepCoSI to evaluate the covalent-ligandability of each cysteine. You can search the profiled database with PDB ID or Uniprot ID in Home Page to check the ligandability of each cysteine for a specific PDB structure or protein.
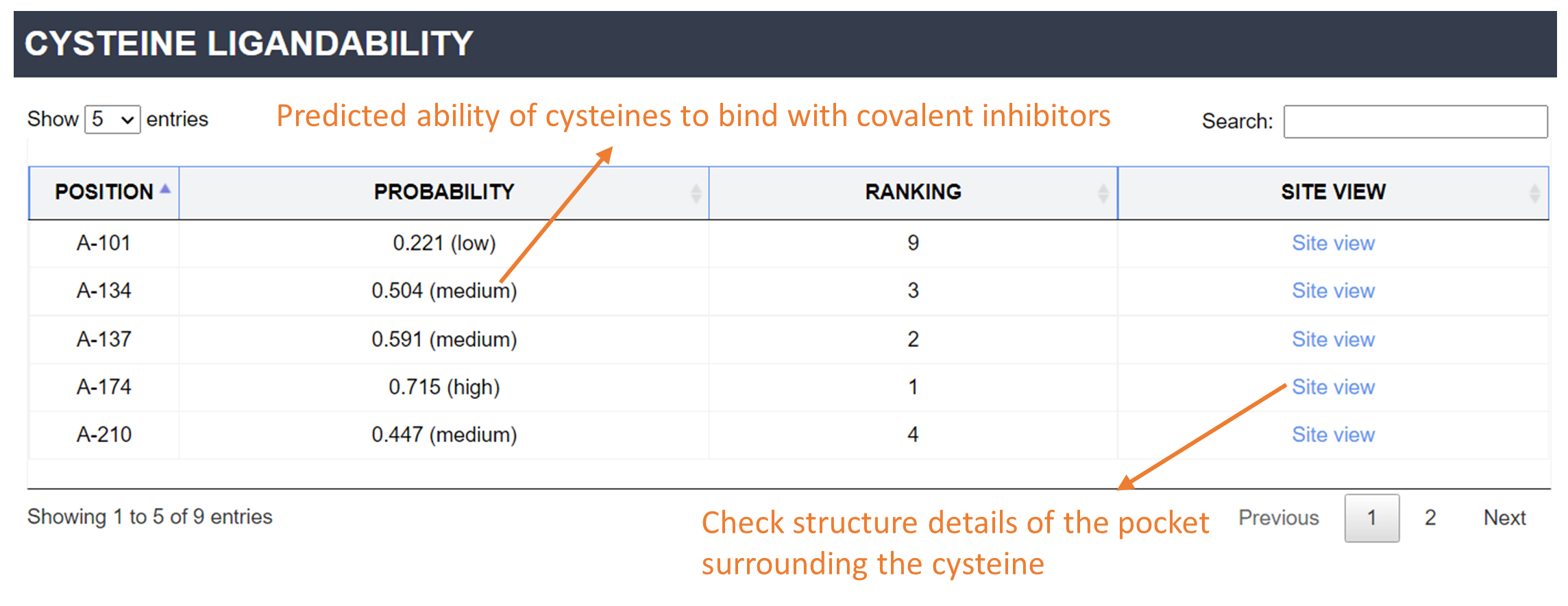
Ability to bind with a covalent inhibitor: For each structure, DeepCoSI encoded and processed the characterization of pocket and the interactions between each flexible cysteine and the surrounding environment. Cysteines were ranked and tagged with 'high', 'medium' and 'low', based on their prediction value (the ability to bind with a covalent inhibitor). You can click 'Site View' to check structure details of the pocket surrounding the cysteine.
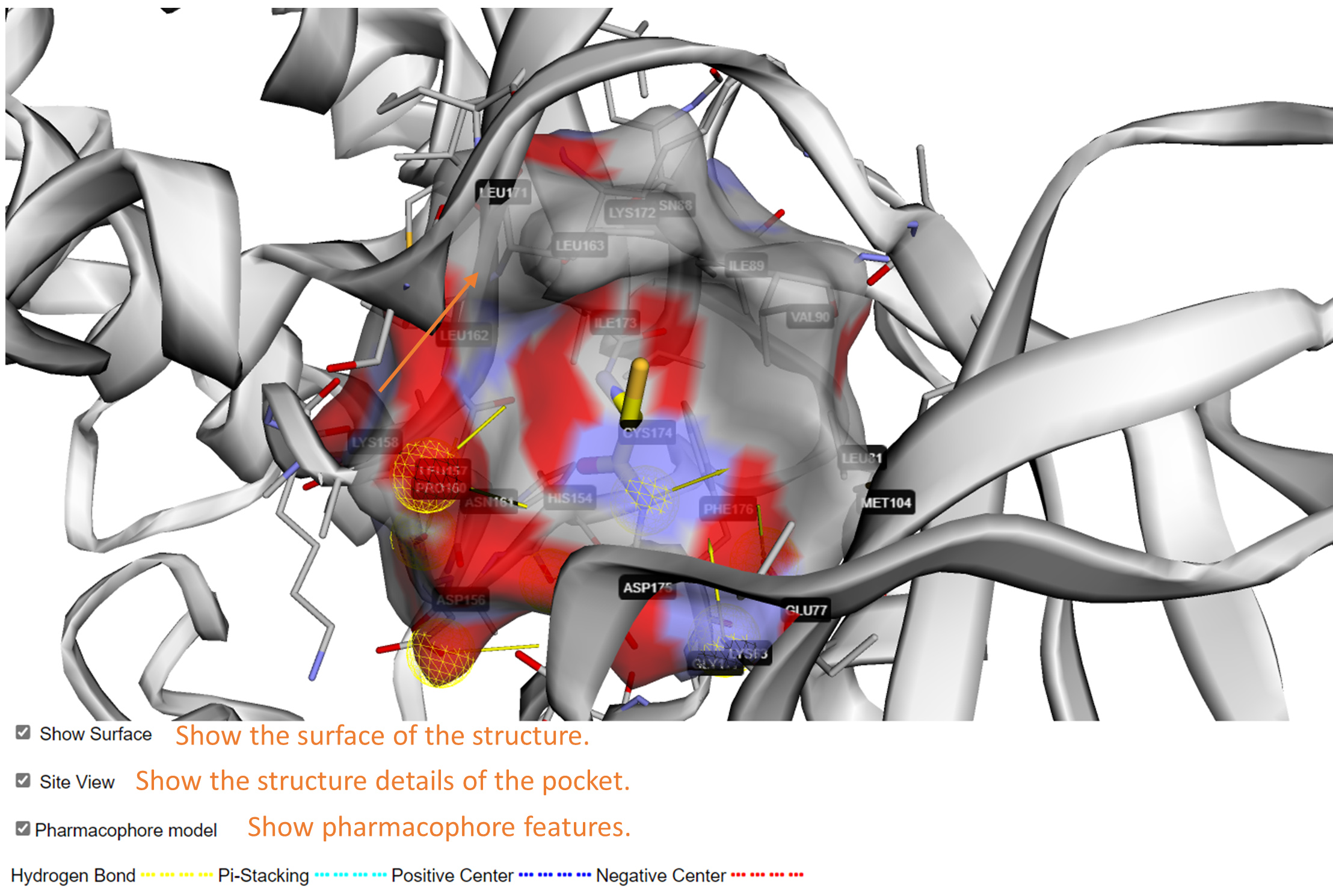
Site view: You can check the structure of predicted covalent binding site in this page. Please check 'Site View' to show details of the pocket surrounding the cysteine. The amino acids within 5 Å around the cysteine are shown as stick. A semitransparent surface will be added to the whole structure or the site if you check the 'Show Surface'. You can check the pharmacophore features around the pocket by checking 'Pharmacophore model', which are dedicated to provide useful information for rational design of targeted covalent inhibitors for the site.
✔ Natural Products and Covalent Virtual Screening Library:
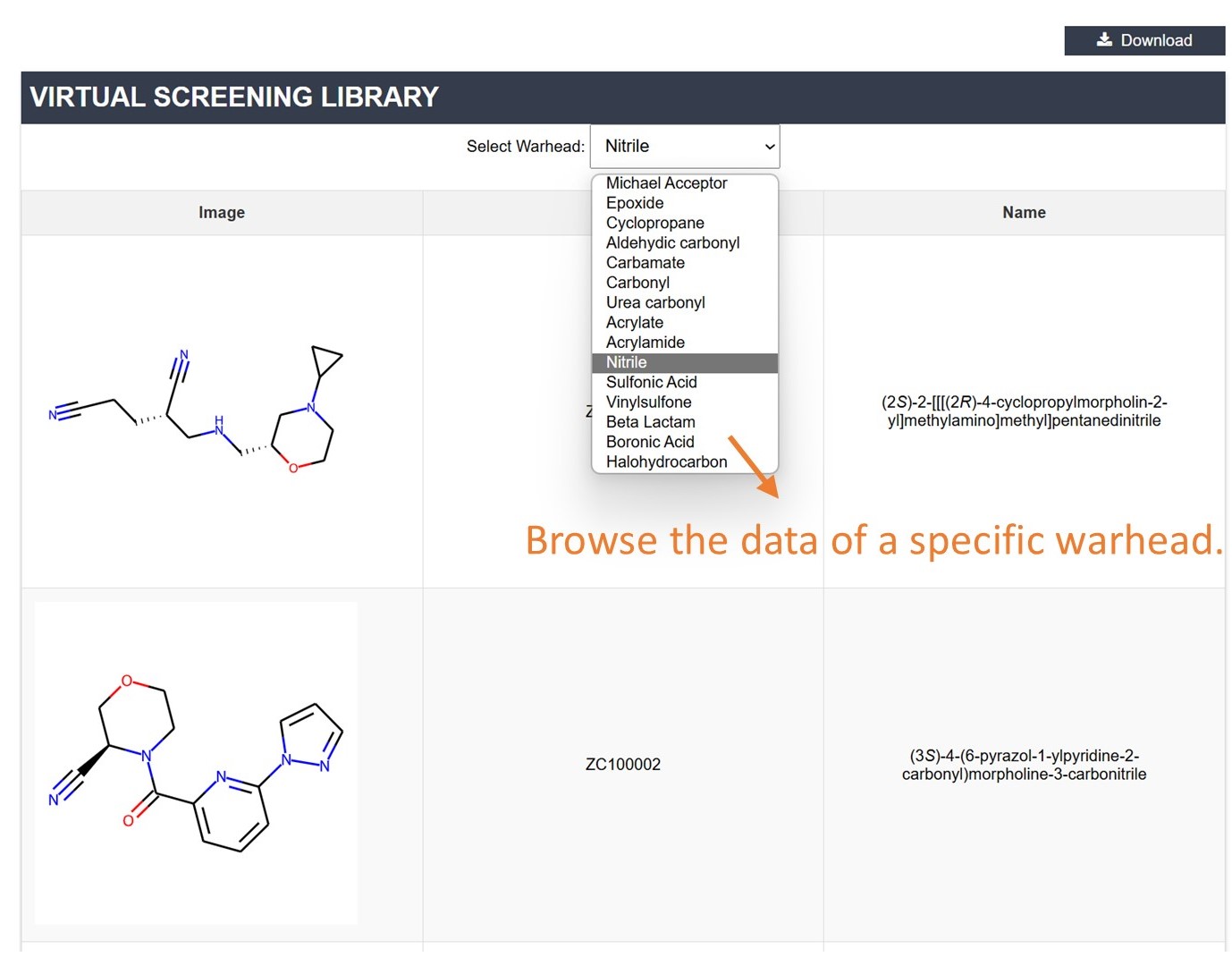
You can choose specific warhead categories to view related natural products with covalent binding potential or compounds in the covalent virtual screening library.
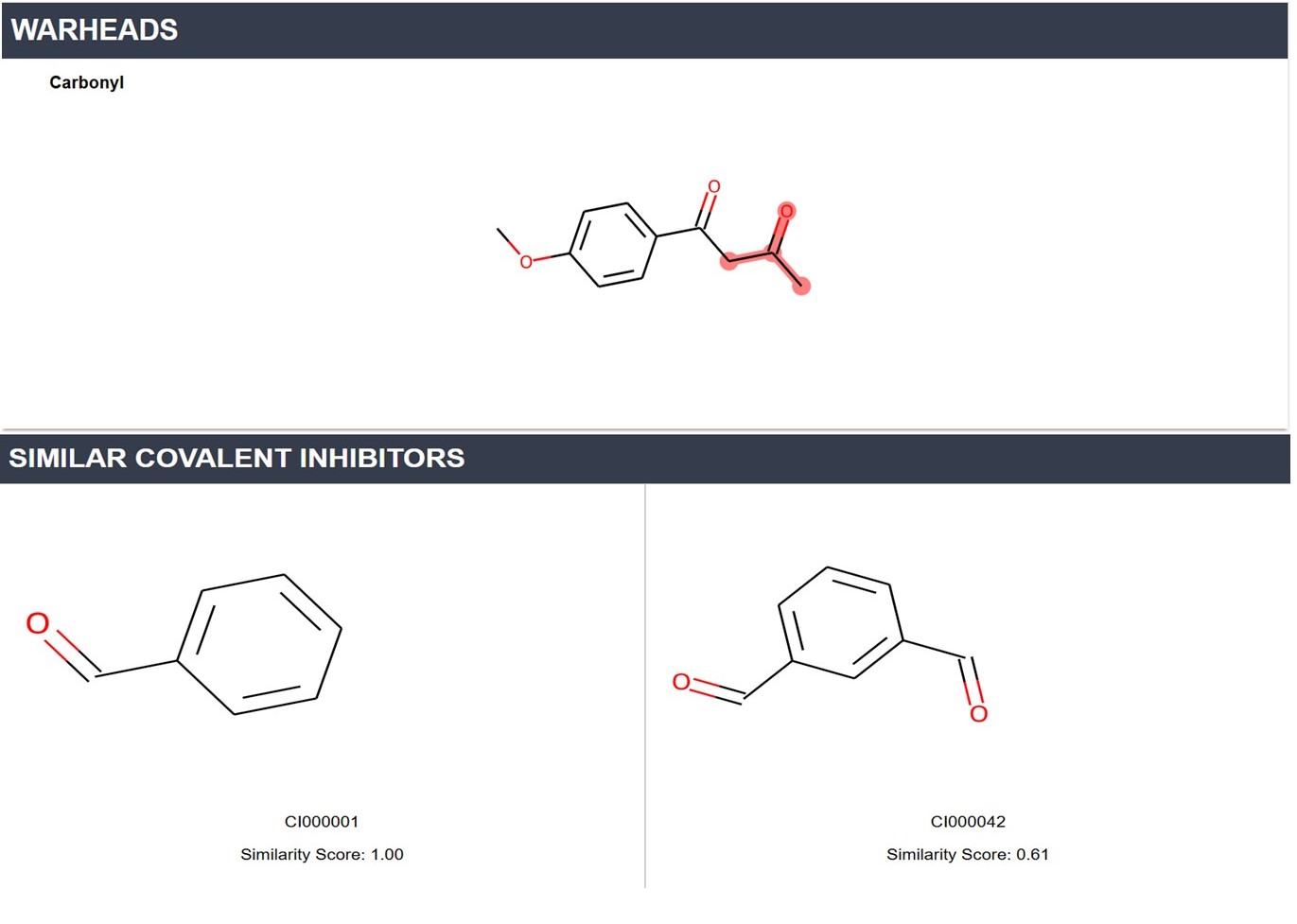
Each compound page provides information including the InChI, InChI key, IUPAC name, and a highlight of all covalent warhead substructures. Additionally, we offer 53 ADMET properties and 10 physicochemical properties predicted by ADMET 2.0. For interpretation of these properties, please refer to the ADMET 2.0 documentation.
Furthermore, this page lists covalent inhibitors and covalent drugs with structures similar to the compound (structural similarity greater than 0.5).
Structural characteristics of compounds with various warhead types across two compound libraries:
✔ Data Upload Feature:
To contribute your own data, click the 'Deposit' button in the navigation bar. This feature offers two submission methods: single molecule mode and multi-molecule mode.
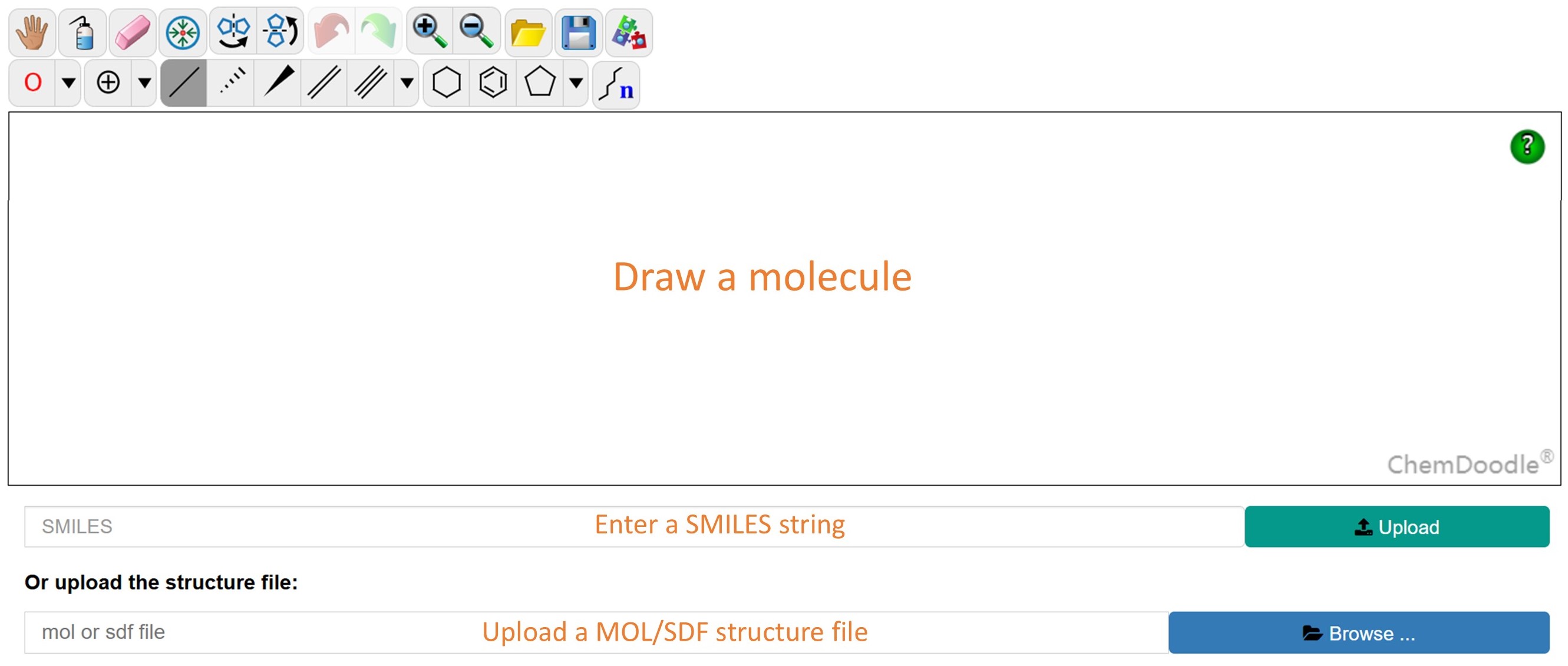
In single molecule mode, you can define the chemical structure by drawing it, pasting a SMILES string, or uploading a structure file. You may also submit five types of activity data: 'Target Inhibition' to describe covalent inhibition effects, 'Bioactivity' for cellular or organism-level activity, 'Selectivity' indicating inhibitory capacity on other targets, 'ADMET' properties, and 'Reactivity' for intrinsic covalent reactivity, often measured by the compound's interaction with glutathione.
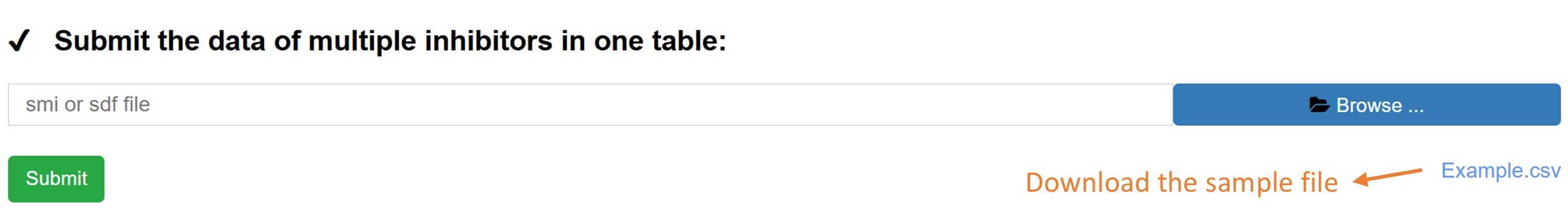
The multi-molecule mode enables you to upload data for multiple compounds at once using a tabular format. An example file is available for guidance. All user-submitted data are subject to a thorough manual review to ensure accuracy and quality before being incorporated into the database.
For more help, please feel free to contact us. You can get the contact information on the Contact Page.
Use Cases
✔ Case 1: Optimization and warhead selection for medicinal chemists. (Take BTK for example)
Step 1: By searching with the keyword ‘BTK’, you can easily get to the ‘BTK Page’.
Step 2: The first useful information is the crystal structure of BTK bound to a covalent drug called ibrutinib. The ‘Site View’ allows to recognize the covalent binding nucleophilic residue and residues within 5 Å around the inhibitor and to check the protein-ligand binding mode in the binding site, which provides useful information to the structure-based design of targeted covalent inhibitors.
Step 3: The second useful information is BTK covalent inhibitors published in literatures. Users can know which warhead has been successfully utilized for the design of BTK covalent inhibitor. By comparing the structures of a bunch of analogues and according to their structure-activity relationship, users might know what type of substituent strategy can increase the bioactivity while others not.
Step 4: The downloadable structure and bioactivity data of all inhibitors allow to use a drug design software like Schrödinger to generate a pharmacophore model, which will contribute to rational design of a covalent inhibitor for BTK.
✔ Case 2: Evaluate the performance of covalent docking tools
Step 1: In the ‘Download Page’, it’s easy to download all the data of inhibitors including the structures, bioactivity and warhead information.
Step 2: Docking these inhibitors with different tools.
Step 3: Calculating the correlation between docking score and bioactivity can compare the prediction performance of these computational algorithms.