Futibatinib
Drug information
- CovInDB Drug
- DB15149
- Name
- Futibatinib
- Molecular Formula
- C22H22N6O3
- Molecular Weight
- 418.1753 g/mol
- Description
- Futibatinib is a kinase inhibitor used to treat intrahepatic cholangiocarcinoma in previously treated adults.
- Status
- approved, investigational
- Structure
-
- Indication
- Futibatinib is indicated to treat adults with previously treated, unresectable, locally advanced or metastatic intrahepatic cholangiocarcinoma harbouring fibroblast growth factor receptor 2 (FGFR2) gene fusions or other rearrangements. In Europe, it is indicated in patients whose disease has progressed after at least one prior line of systemic therapy. Futibatinib is approved in the US under accelerated approval and in Europe under conditional marketing authorization. This currently approved indication is subject to change, as it may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
- Mechanism of action
-
Fibroblast Growth Factor receptor (FGFR) pathway play a key role in cell proliferation, differentiation, migration, and survival. Notably, FGFR genomic aberrations and aberrant FGFR signalling pathways are observed in some cancers, as constitutive FGFR signalling can support the proliferation and survival of malignant cells. Futibatinib is a selective, irreversible inhibitor of FGFR 1, 2, 3, and 4 with IC50 values of less than 4 nM. It binds to the FGFR kinase domain by forming a covalent bond with cysteine in the ATP-binding pocket. Upon binding to FGFR, futibatinib blocks FGFR phosphorylation and downstream signalling pathways, such as the RAS-dependent mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase (PI3KCA)/Akt/mTOR, phospholipase Cγ (PLCγ), and JAK/STAT. Futibatinib ultimately decreases cell viability in cancer cell lines with FGFR alterations, including FGFR fusions or rearrangements, amplifications, and mutations.
- IUPAC Name
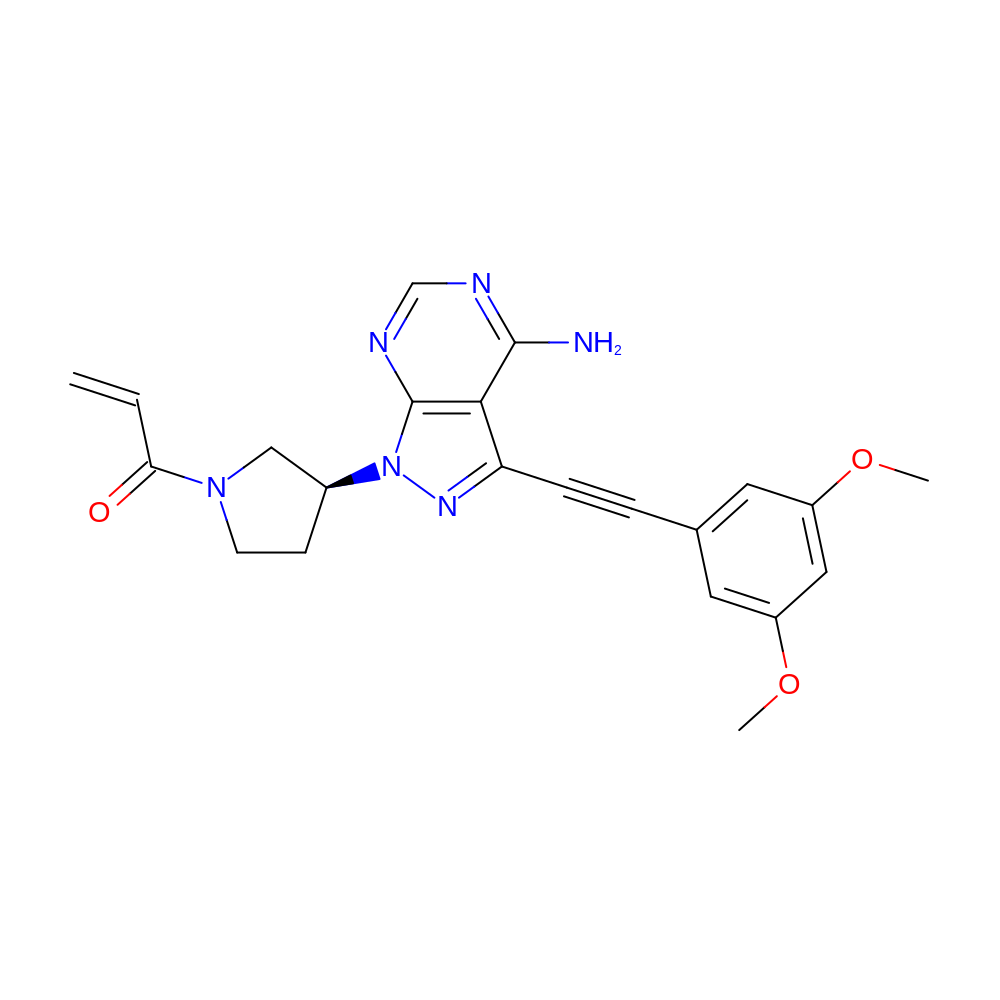
- 1-[(3S)-3-{4-amino-3-[2-(3,5-dimethoxyphenyl)ethynyl]-1H-pyrazolo[3,4-d]pyrimidin-1-yl}pyrrolidin-1-yl]prop-2-en-1-one
- InChI
- InChI=1S/C22H22N6O3/c1-4-19(29)27-8-7-15(12-27)28-22-20(21(23)24-13-25-22)18(26-28)6-5-14-9-16(30-2)11-17(10-14)31-3/h4,9-11,13,15H,1,7-8,12H2,2-3H3,(H2,23,24,25)/t15-/m0/s1
- InChI Key
- KEIPNCCJPRMIAX-HNNXBMFYSA-N
- Canonical SMILES
- COC1=CC(=CC(OC)=C1)C#CC1=NN([C@H]2CCN(C2)C(=O)C=C)C2=C1C(N)=NC=N2
- Reference
- DrugBank
Covalent Inhibition
- Warhead
- Micheal Acceptor
- Target
-
Fibroblast growth factor receptor 1 [ UniProt: P11362 ]
Fibroblast growth factor receptor 2 [ UniProt: P21802 ]
Fibroblast growth factor receptor 3 [ UniProt: P22607 ]
Fibroblast growth factor receptor 4 [ UniProt: P22455 ]
- Site
- CYS-491
- Inhibition Mechanism
-

Discovery of Futibatinib: The First Covalent FGFR Kinase Inhibitor in Clinical Use
3D Structure
Calculated Properties
- logP
-
1.79
Computed by ALOGPS
- logS
-
-4.02
Computed by ALOGPS
- Heavy Atom Count
-
31
Computed by RDKit
- Ring Count
-
4
Computed by RDKit
- Hydrogen Bond Acceptor Count
-
8
Computed by RDKit
- Hydrogen Bond Donor Count
-
1
Computed by RDKit
- Rotatable Bond Count
-
4
Computed by RDKit
- Topological Polar Surface Area
-
108.39 Å2
Computed by RDKit
Similar compounds in Virtual Screening library
Similar Natural compounds
No similar natural compounds found for this drug.