Zanubrutinib
Drug information
- CovInDB Drug
- DB15035
- Name
- Zanubrutinib
- Molecular Formula
- C27H29N5O3
- Molecular Weight
- 471.23 g/mol
- Description
- Zanubrutinib is a novel Bruton's tyrosine kinase (BTK) inhibitor used for the treatment of adult patients with mantle cell lymphoma (MCL) who have received at least one prior therapy.Mantle cell lymphoma is an aggressive mature B-cell non-Hodgkin lymphoma that is associated with early relapse, poor clinical outcomes, and long-term survival.BTK is an enzyme that plays a role in oncogenic signalling pathways, where it promotes the survival and proliferation of malignant B cells.Compared to the first-generation BTK inhibitor Due to this enhanced selectivity towards BTK, zanubrutinib belongs to the second-generation BTK inhibitor drug group that also includes which measures the proportion of patients in a trial whose tumour is entirely or partially destroyed by a drug.It is currently marketed under the trade name BRUKINSA and is available as oral capsules.
- Status
- approved, investigational
- Structure
-
- Indication
- Zanubrutinib is indicated for the treatment of adult patients with mantle cell lymphoma (MCL) who have received at least one prior therapy.
- Mechanism of action
-
Bruton's tyrosine kinase (BTK) is a non-receptor kinase and a signalling molecule for the B cell receptors expressed on the peripheral B cell surface.The BCR signalling pathway plays a crucial role in normal B-cell development but also the proliferation and survival of malignant B cells in many B cell malignancies, including mantle-cell lymphoma (MCL).Once activated by upstream Src-family kinases, BTK phosphorylates phospholipase-Cγ (PLCγ), leading to Ca2+ mobilization and activation of NF-κB and MAP kinase pathways. These downstream cascades promote the expression of genes involved in B cell proliferation and survival.The BCR signalling pathway also induces the anti-apoptotic protein Bcl-xL and regulates the integrin α4β1 (VLA-4)-mediated adhesion of B cells to vascular cell adhesion molecule-1 (VCAM-1) and fibronectin via BTK. Apart from the direct downstream signal transduction pathway of B cells, BTK is also involved in chemokine receptor, Toll-like receptor (TLR) and Fc receptor signalling pathways.
Zanubrutinib inhibits BTK by forming a covalent bond with cysteine 481 residue in the adenosine triphosphate (ATP)–binding pocket of BTK, which is the enzyme's active site. This binding specificity is commonly seen with other BTK inhibitors. Due to this binding profile, zanubrutinib may also bind with varying affinities to related and unrelated ATP-binding kinases that possess a cysteine residue at this position.By blocking the BCR signalling pathway, zanubrutinib inhibits the proliferation, trafficking, chemotaxis, and adhesion of malignant B cells, ultimately leading to reduced tumour size.Zanubrutinib was also shown to downregulate programmed death-ligand 1 (PD-1) expression and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) on CD4+ T cells. - IUPAC Name
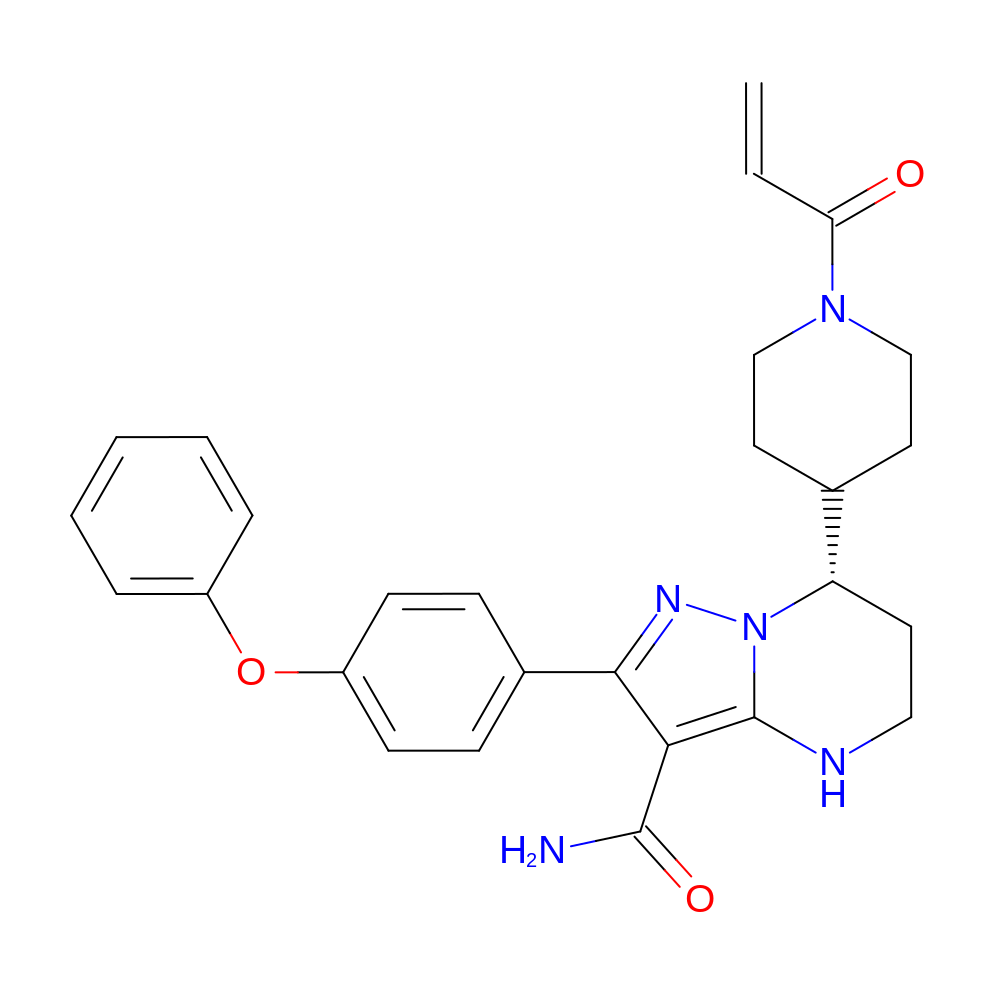
- (7S)-2-(4-phenoxyphenyl)-7-(1-prop-2-enoyl-4-piperidyl)-4,5,6,7-tetrahydropyrazolo[1,5-a]pyrimidine-3-carboxamide
- InChI
- InChI=1S/C27H29N5O3/c1-2-23(33)31-16-13-18(14-17-31)22-12-15-29-27-24(26(28)34)25(30-32(22)27)19-8-10-21(11-9-19)35-20-6-4-3-5-7-20/h2-11,18,22,29H,1,12-17H2,(H2,28,34)/t22-/m0/s1
- InChI Key
- RNOAOAWBMHREKO-QFIPXVFZSA-N
- Canonical SMILES
- NC(=O)C1=C2NCC[C@@H](C3CCN(CC3)C(=O)C=C)N2N=C1C1=CC=C(OC2=CC=CC=C2)C=C1
- Reference
- DrugBank
Covalent Inhibition
- Warhead
- Micheal Acceptor
- Target
-
Tyrosine-protein kinase BTK [ UniProt: Q06187 ]
- Site
- CYS-481
- Inhibition Mechanism
-
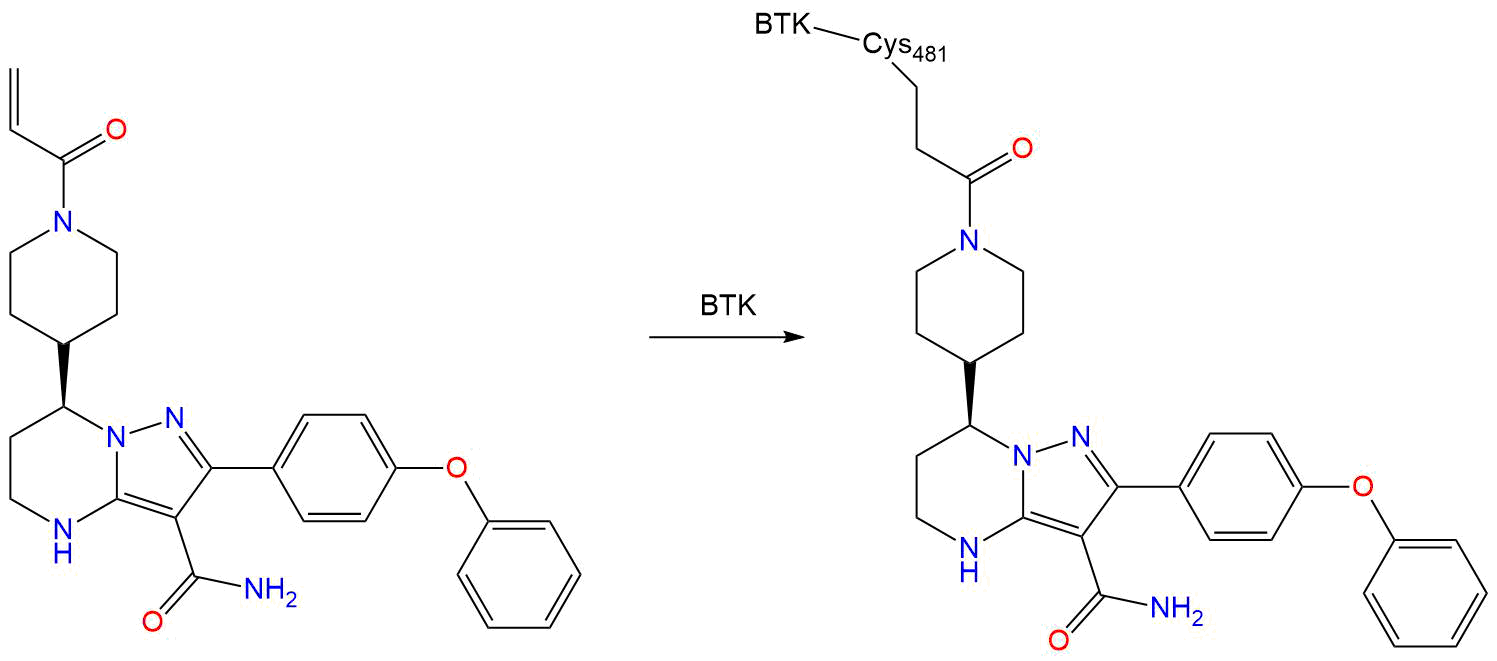
Discovery of Zanubrutinib (BGB-3111), a Novel, Potent, and Selective Covalent Inhibitor of Bruton's Tyrosine Kinase
3D Structure
Calculated Properties
- logP
-
3.27
Computed by ALOGPS
- logS
-
-4.66
Computed by ALOGPS
- Heavy Atom Count
-
35
Computed by RDKit
- Ring Count
-
5
Computed by RDKit
- Hydrogen Bond Acceptor Count
-
6
Computed by RDKit
- Hydrogen Bond Donor Count
-
2
Computed by RDKit
- Rotatable Bond Count
-
6
Computed by RDKit
- Topological Polar Surface Area
-
102.48 Å2
Computed by RDKit
Similar compounds in Virtual Screening library
Similar Natural compounds
No similar natural compounds found for this drug.