Dacomitinib
Drug information
- CovInDB Drug
- DB11963
- Name
- Dacomitinib
- Molecular Formula
- C24H25ClFN5O2
- Molecular Weight
- 469.17 g/mol
- Description
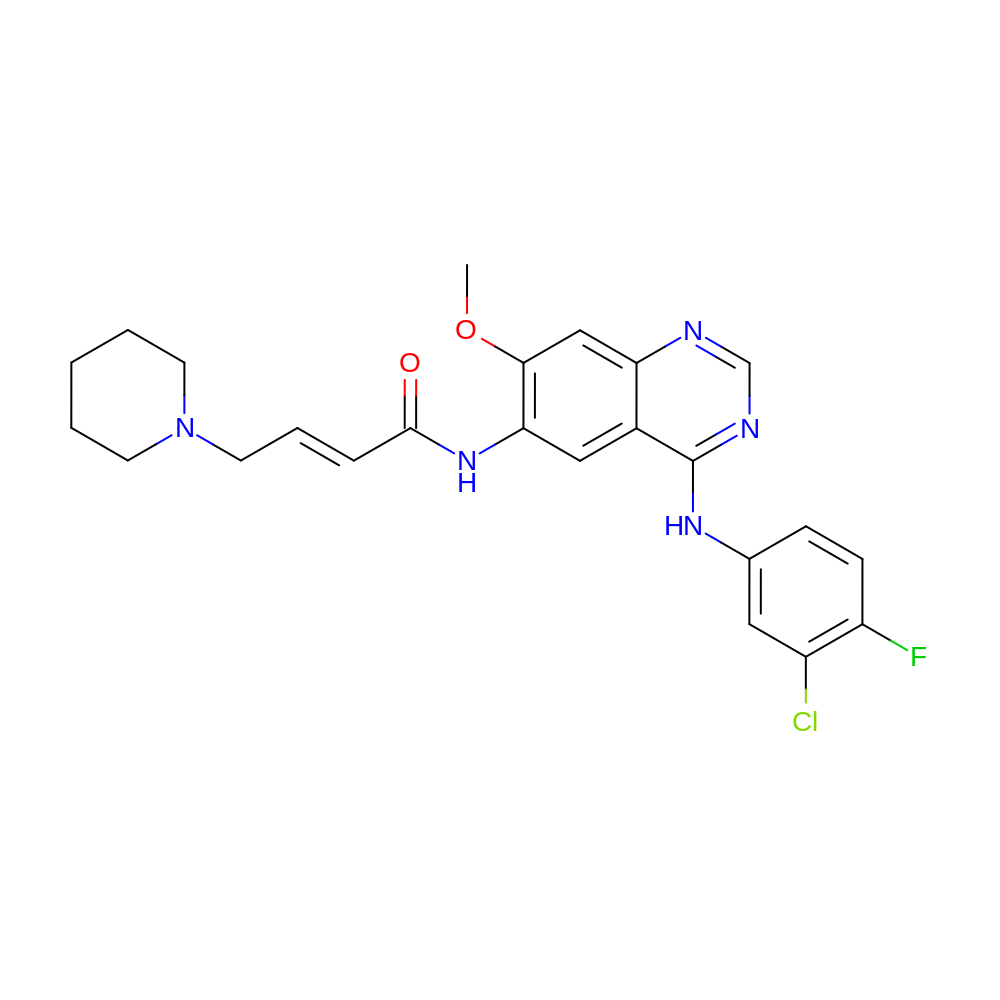
- Dacomitinib, designed as (2E)-N-16-4-(piperidin-1-yl) but-2-enamide, is an oral highly selective quinazalone part of the second-generation tyrosine kinase inhibitors which are characterized by the irreversible binding at the ATP domain of the epidermal growth factor receptor family kinase domains.Some evidence in the literature suggests the therapeutic potential of dacomitinib in the epithelial ovarian cancer model, although further investigations are needed.
- Status
- approved, investigational
- Structure
-
- Indication
- Dacomitinib is indicated as the first-line treatment of patients with metastatic non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 19 deletion or exon 21 L858R substitution mutations as verified by an FDA-approved test.
Lung cancer is the leading cause of cancer death and NSCLC accounts for 85% of lung cancer cases. From the cases of NSCLC, approximately 75% of the patients present a late diagnosis with metastatic and advanced disease which produces a survival rate of 5%. The presence of a mutation in EGFR accounts for more than the 60% of the NSCLC cases and the overexpression of EGFR is associated with frequent lymph node metastasis and poor chemosensitivity. - Mechanism of action
-
Dacomitinib is an irreversible small molecule inhibitor of the activity of the human epidermal growth factor receptor (EGFR) family (EGFR/HER1, HER2, and HER4) tyrosine kinases. It achieves irreversible inhibition via covalent bonding to the cysteine residues in the catalytic domains of the HER receptors.The affinity of dacomitinib has been shown to have an IC50 of 6 nmol/L.Around 40% of cases show amplification of EGFR gene and 50% of the cases present the EGFRvIII mutation which represents a deletion that produces a continuous activation of the tyrosine kinase domain of the receptor.
- IUPAC Name
- (E)-N-[4-(3-chloro-4-fluoro-anilino)-7-methoxy-quinazolin-6-yl]-4-(1-piperidyl)but-2-enamide
- InChI
- InChI=1S/C24H25ClFN5O2/c1-33-22-14-20-17(24(28-15-27-20)29-16-7-8-19(26)18(25)12-16)13-21(22)30-23(32)6-5-11-31-9-3-2-4-10-31/h5-8,12-15H,2-4,9-11H2,1H3,(H,30,32)(H,27,28,29)/b6-5+
- InChI Key
- LVXJQMNHJWSHET-AATRIKPKSA-N
- Canonical SMILES
- COC1=C(NC(=O)\C=C\CN2CCCCC2)C=C2C(NC3=CC(Cl)=C(F)C=C3)=NC=NC2=C1
- Reference
- DrugBank
Covalent Inhibition
- Warhead
- Micheal Acceptor
- Target
-
Epidermal growth factor receptor [ UniProt: P00533 ]
- Site
- CYS-797
- Inhibition Mechanism
-

Insights into the aberrant activity of mutant EGFR kinase domain and drug recognition
3D Structure
Calculated Properties
- logP
-
4.88
Computed by ALOGPS
- logS
-
-4.73
Computed by ALOGPS
- Heavy Atom Count
-
33
Computed by RDKit
- Ring Count
-
4
Computed by RDKit
- Hydrogen Bond Acceptor Count
-
6
Computed by RDKit
- Hydrogen Bond Donor Count
-
2
Computed by RDKit
- Rotatable Bond Count
-
7
Computed by RDKit
- Topological Polar Surface Area
-
79.38 Å2
Computed by RDKit
Similar compounds in Virtual Screening library
Similar Natural compounds
No similar natural compounds found for this drug.