Osimertinib
Drug information
- CovInDB Drug
- DB09330
- Name
- Osimertinib
- Molecular Formula
- C28H33N7O2
- Molecular Weight
- 499.27 g/mol
- Description
- Osimertinib is an oral, third-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) drug developed by AstraZeneca Pharmaceuticals. Its use is indicated for the treatment of metastatic non-small cell lung cancer (NSCLC) in cases where tumour EGFR expression is positive for the T790M mutation as detected by FDA-approved testing and which has progressed following therapy with a first-generation EGFR tyrosine kinase inhibitor. Approximately 10% of patients with NSCLC have a rapid and clinically effective response to EGFR-TKIs due to the presence of specific activating EGFR mutations within the tumour cells. More specifically, deletions around the LREA motif in exon 19 and exon 21 L858R point mutations are correlated with response to therapy. Development of third-generation EGFR-TKIs, such as osimertinib, has been in response to altered tumour resistance patterns following treatment and toxic side effects that impact patient quality of life. Treatment with first-generation EGFR-TKIs (gefitinib and erlotinib) has been associated with the development of resistance through activating mutations in the EGFR gene. Second-generation EGFR-TKIs (afatinib and dacomitinib) were then developed to be more potent inhibitors, although their use is associated with increased toxicity through nonspecific targeting of wild-type EGFR. In contrast, third-generation inhibitors are specific for the gate-keeper T790M mutations which increases ATP binding activity to EGFR and result in poor prognosis for late-stage disease. Furthermore, osimertinib has been shown to spare wild-type EGFR during therapy, thereby reducing non-specific binding and limiting toxicity.
- Status
- approved
- Structure
-
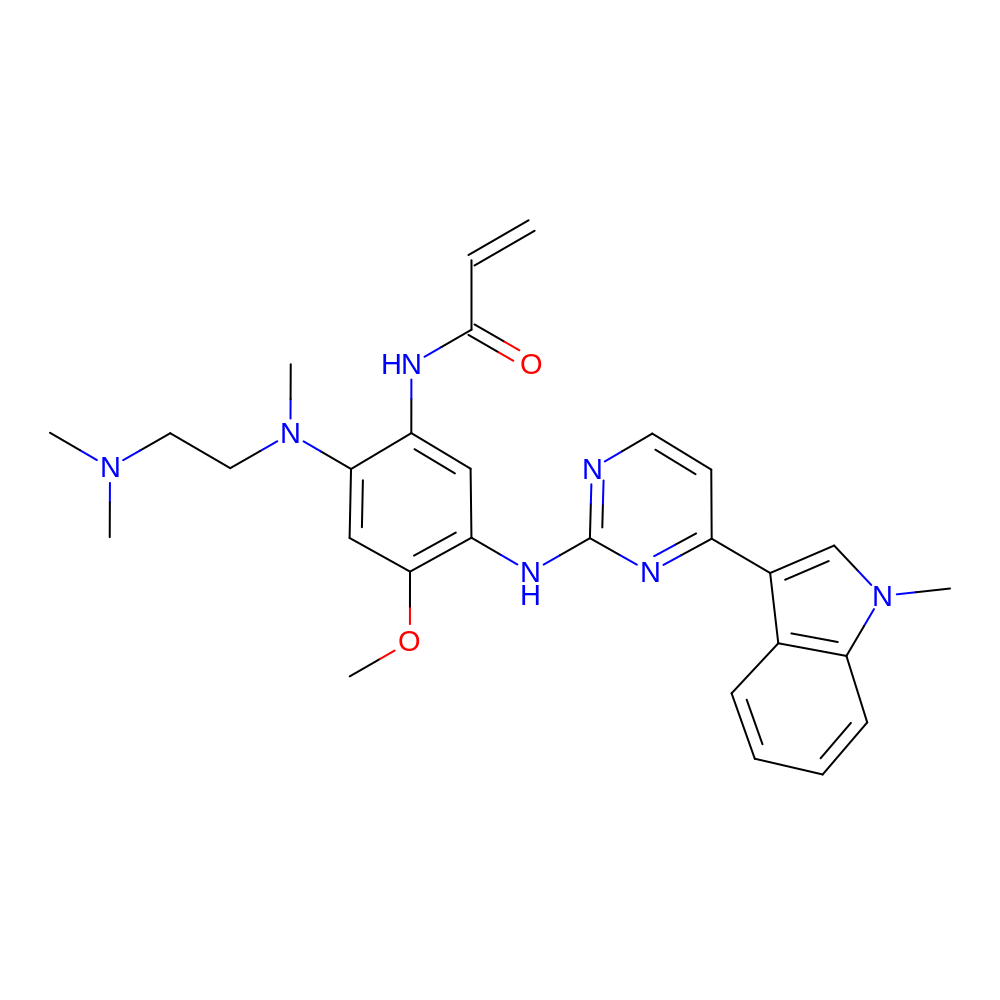
- Indication
- Osimertinib is indicated for the treatment of patients with metastatic epidermal growth factor receptor (EGFR) T790M mutation-positive non-small cell lung cancer (NSCLC), as detected by an FDA- approved test, who have progressed on or after EGFR-TKI therapy.
- Mechanism of action
-
Osimertinib is an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) that binds to certain mutant forms of EGFR (T790M, L858R, and exon 19 deletion) that predominate in non-small cell lung cancer (NSCLC) tumours following treatment with first-line EGFR-TKIs. As a third-generation tyrosine kinase inhibitor, osimertinib is specific for the gate-keeper T790M mutation which increases ATP binding activity to EGFR and results in poor prognosis for late-stage disease. Furthermore, osimertinib has been shown to spare wild-type EGFR during therapy, thereby reducing non-specific binding and limiting toxicity.
- IUPAC Name
- N-[2-[2-(dimethylamino)ethyl-methyl-amino]-4-methoxy-5-[[4-(1-methylindol-3-yl)pyrimidin-2-yl]amino]phenyl]prop-2-enamide
- InChI
- InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32)
- InChI Key
- DUYJMQONPNNFPI-UHFFFAOYSA-N
- Canonical SMILES
- COC1=C(NC2=NC=CC(=N2)C2=CN(C)C3=C2C=CC=C3)C=C(NC(=O)C=C)C(=C1)N(C)CCN(C)C
- Reference
- DrugBank
Covalent Inhibition
- Warhead
- Micheal Acceptor
- Target
-
Epidermal growth factor receptor [ UniProt: P00533 ]
- Site
- CYS-797
- Inhibition Mechanism
-
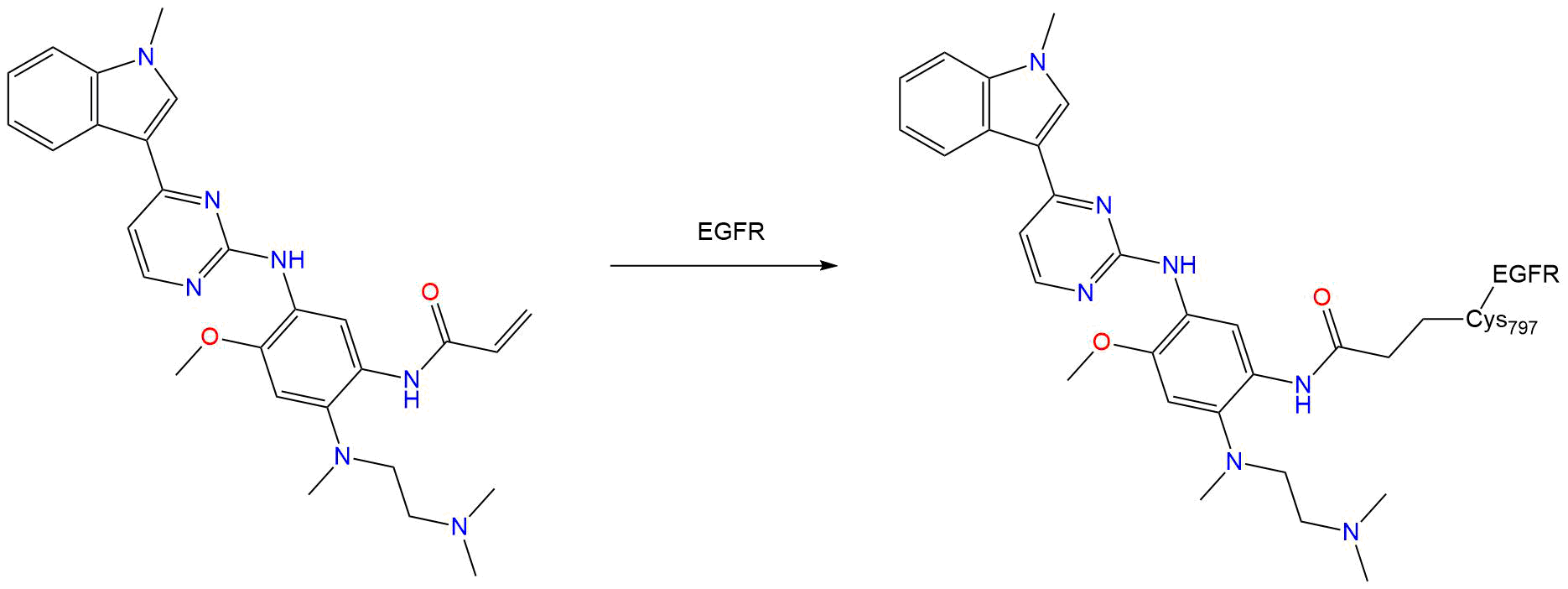
Binding mode of the breakthrough inhibitor AZD9291 to epidermal growth factor receptor revealed
3D Structure
Calculated Properties
- logP
-
4.47
Computed by ALOGPS
- logS
-
-4.35
Computed by ALOGPS
- Heavy Atom Count
-
37
Computed by RDKit
- Ring Count
-
4
Computed by RDKit
- Hydrogen Bond Acceptor Count
-
8
Computed by RDKit
- Hydrogen Bond Donor Count
-
2
Computed by RDKit
- Rotatable Bond Count
-
10
Computed by RDKit
- Topological Polar Surface Area
-
87.55 Å2
Computed by RDKit
Similar compounds in Virtual Screening library
Similar Natural compounds
No similar natural compounds found for this drug.