Ibrutinib
Drug information
- CovInDB Drug
- DB09053
- Name
- Ibrutinib
- Molecular Formula
- C25H24N6O2
- Molecular Weight
- 440.2 g/mol
- Description
- Ibrutinib is a small molecule that acts as an irreversible potent inhibitor of Burton's tyrosine kinase. It is designated as a targeted covalent drug and it presents a very promising activity in B cell malignancies.Ibrutinib was developed by Pharmacyclics Inc and in November 2013 was FDA-approved for the treatment of mantle cell lymphoma. Later, in February 2014, ibrutinib was approved for the treatment of chronic lymphocytic leukemia and it is also indicated for the treatment of patients with Waldenstr?m's Macroglobulinemia.Ibrutinib has also been approved by the EMA for the treatment of chronic lymphocytic leukemia and mantle cell lymphoma.Ibrutinib was approved for use in chronic graft versus host disease in August 2017 .
- Status
- approved
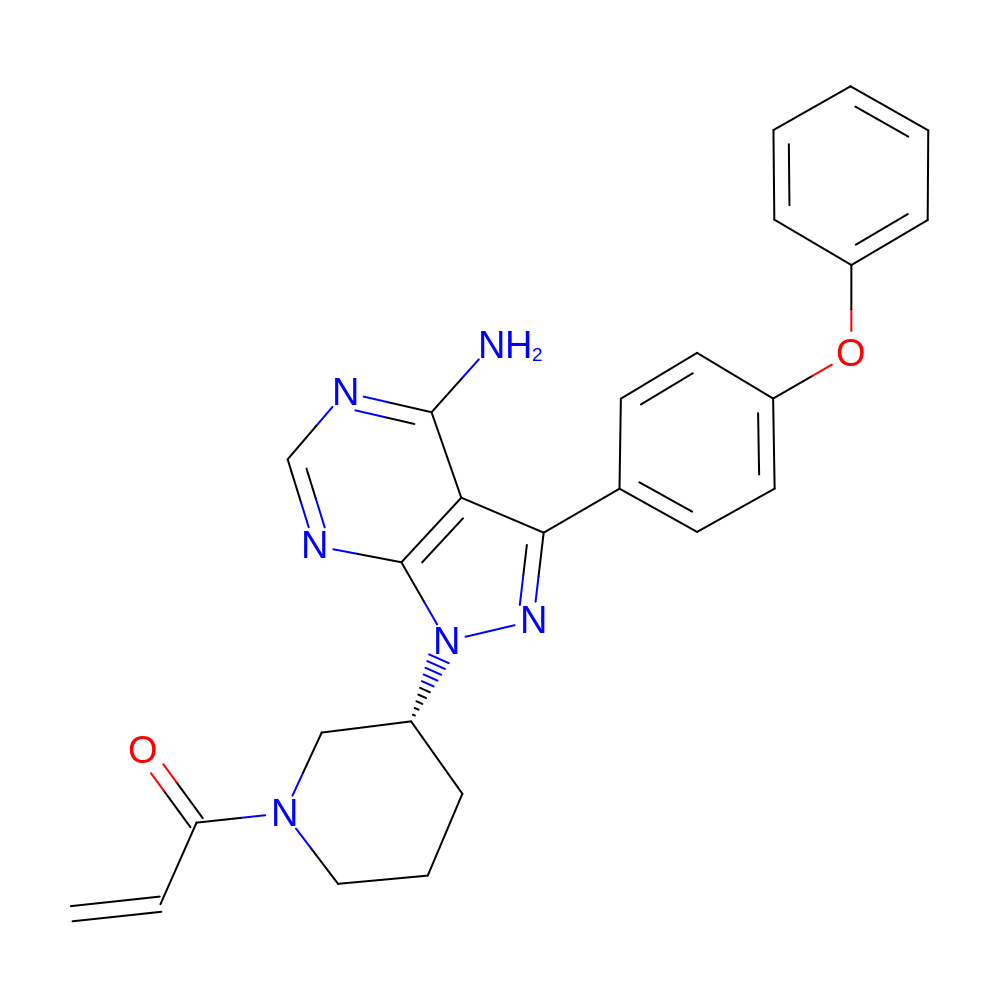
- Structure
-
- Indication
- Ibrutinib acquired an accelerated approval for the treatment of mantle cell lymphoma who have received at least one prior therapy.Mantle cell lymphoma (MCL) is a B-cell non-Hodgkin lymphoma that develops in the outer edge of a lymph node. MCL is usually diagnosed at late stages and it is easily spread into bone marrow, spleen, liver and gastrointestinal tract.CLL is a type of cancer caused by an overproduction of lymphocytes by the bone marrow. Some of the symptoms include swollen lymph nodes and tiredness.CLL with 17p is a type of leukemia in which a deletion in 17p disrupts the tumor suppressor p53 by deleting one allele of the TP53 gene. The remaining allele is mainly inactivated and thus, this type of leukemia is unresponsive to p53-dependent treatments.WM, also called lymphoplasmacytic lymphoma, is a type of non-Hodgkin lymphoma in which the cancer cells make large amounts of macroglobulin. The macroglobulin is a monoclonal protein that corresponds to the type of IgM antibodies and the unrestricted formation of this protein causes typical symptoms such as excessive bleeding and effects in vision and nervous system.
- Mechanism of action
-
Ibrutinib is an inhibitor of Bruton’s tyrosine kinase (BTK). It forms a covalent bond with a cysteine residue in the active site of BTK (Cys481), leading to its inhibition. The inhibition of BTK plays a role in the B-cell receptor signaling and thus, the presence of ibrutinib prevents the phosphorylation of downstream substrates such as PLC-γ.
- IUPAC Name
- 1-[(3R)-3-[4-amino-3-(4-phenoxyphenyl)pyrazolo[3,4-d]pyrimidin-1-yl]-1-piperidyl]prop-2-en-1-one
- InChI
- InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1
- InChI Key
- XYFPWWZEPKGCCK-GOSISDBHSA-N
- Canonical SMILES
- NC1=NC=NC2=C1C(=NN2[C@@H]1CCCN(C1)C(=O)C=C)C1=CC=C(OC2=CC=CC=C2)C=C1
- Reference
- DrugBank
Covalent Inhibition
- Warhead
- Micheal Acceptor
- Target
-
Tyrosine-protein kinase BTK [ UniProt: Q06187 ]
- Site
- CYS-481
- Inhibition Mechanism
-
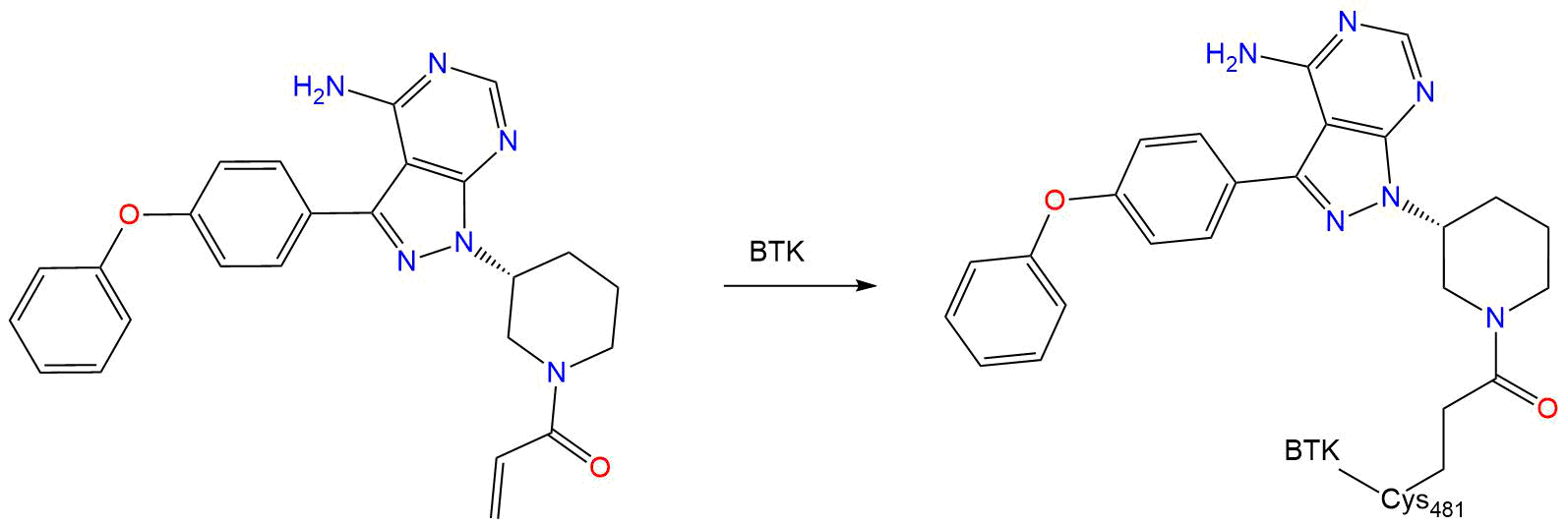
Ability of Bruton's Tyrosine Kinase Inhibitors to Sequester Y551 and Prevent Phosphorylation Determines Potency for Inhibition of Fc Receptor but not B-Cell Receptor Signaling
3D Structure
Calculated Properties
- logP
-
2.76
Computed by ALOGPS
- logS
-
-4.34
Computed by ALOGPS
- Heavy Atom Count
-
33
Computed by RDKit
- Ring Count
-
5
Computed by RDKit
- Hydrogen Bond Acceptor Count
-
7
Computed by RDKit
- Hydrogen Bond Donor Count
-
1
Computed by RDKit
- Rotatable Bond Count
-
5
Computed by RDKit
- Topological Polar Surface Area
-
99.16 Å2
Computed by RDKit
Similar compounds in Virtual Screening library
Similar Natural compounds
No similar natural compounds found for this drug.