Afatinib
Drug information
- CovInDB Drug
- DB08916
- Name
- Afatinib
- Molecular Formula
- C24H25ClFN5O3
- Molecular Weight
- 485.16 g/mol
- Description
- Afatinib is a 4-anilinoquinazoline tyrosine kinase inhibitor in the form of a dimaleate salt available as Boehringer Ingelheim's brand name Gilotrif . For oral use, afatinib tablets are a first-line (initial) treatment for patients with metastatic non-small cell lung cancer (NSCLC) with common epidermal growth factor receptor (EGFR) mutations as detected by an FDA-approved test . Gilotrif (afatinib) is the first FDA-approved oncology product from Boehringer Ingelheim .
- Status
- approved
- Structure
-
- Indication
- Afatinib is a kinase inhibitor indicated as monotherapy for the first-line treatment of (a) Epidermal Growth Factor Receptor (EGFR) TKI (tyrosine kinase inhibitor)-naive adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) whose tumours have non-resistant EGFR mutations as detected by an FDA-approved test , and (b) adult patients with locally advanced or metastatic NSCLC of squamous histology progressing on or after platinum-based chemotherapy .
Recently, as of January 2018, the US FDA approved a supplemental New Drug Application for Boehringer Ingelheim's Gilotrif (afatinib) for the first line treatment of patients with metastatic non-small cell lung cancer (NSCLC) whose tumors have non-resistant epidermal growth factor receptor (EGFR) mutations as detected by an FDA-approved test . The new label includes data on three additional EGFR mutations: L861Q, G719X and S768I . - Mechanism of action
-
Afatinib is a potent and selective, irreversible ErbB family blocker . Afatinib covalently binds to and irreversibly blocks signaling from all homo and heterodimers formed by the ErbB family members EGFR (ErbB1), HER2 (ErbB2), ErbB3 and ErbB4 .
In particular, afatinib covalently binds to the kinase domains of EGFR (ErbB1), HER2 (ErbB2), and HER4 (ErbB4) and irreversibly inhibits tyrosine kinase autophosphorylation, resulting in downregulation of ErbB signaling . Certain mutations in EGFR, including non-resistant mutations in its kinase domain, can result in increased autophosphorylation of the receptor, leading to receptor activation, sometimes in the absence of ligand binding, and can support cell proliferation in NSCLC . Non-resistant mutations are defined as those occurring in exons constituting the kinase domain of EGFR that lead to increased receptor activation and where efficacy is predicted by 1) clinically meaningful tumor shrinkage with the recommended dose of afatinib and/or 2) inhibition of cellular proliferation or EGFR tyrosine kinase phosphorylation at concentrations of afatinib sustainable at the recommended dosage according to validated methods . The most commonly found of these mutations are exon 21 L858R substitutions and exon 19 deletions .
Moreover, afatinib demonstrated inhibition of autophosphorylation and/or in vitro proliferation of cell lines expressing wild-type EGFR and in those expressing selected EGFR exon 19 deletion mutations, exon 21 L858R mutations, or other less common non-resistant mutations, at afatinib concentrations achieved in patients . In addition, afatinib inhibited in vitro proliferation of cell lines overexpressing HER2 . - IUPAC Name
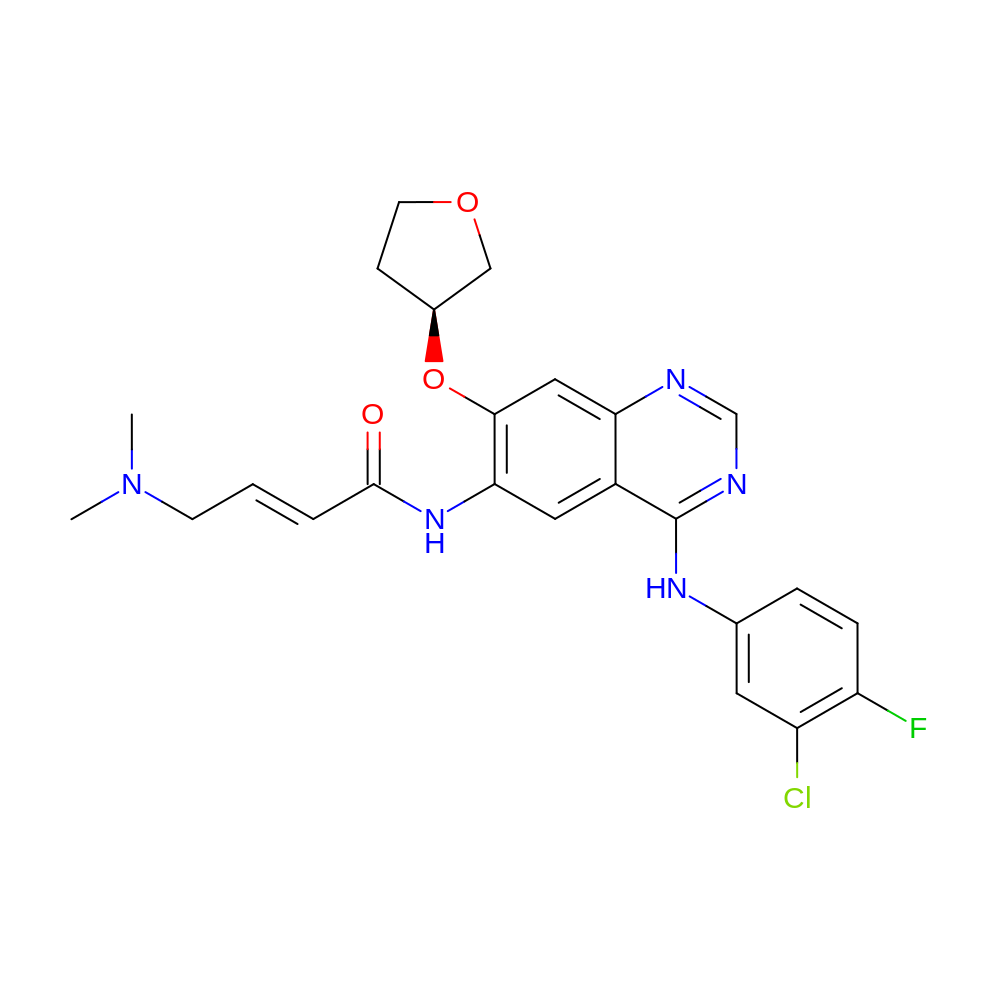
- (E)-N-[4-(3-chloro-4-fluoro-anilino)-7-[(3S)-tetrahydrofuran-3-yl]oxy-quinazolin-6-yl]-4-(dimethylamino)but-2-enamide
- InChI
- InChI=1S/C24H25ClFN5O3/c1-31(2)8-3-4-23(32)30-21-11-17-20(12-22(21)34-16-7-9-33-13-16)27-14-28-24(17)29-15-5-6-19(26)18(25)10-15/h3-6,10-12,14,16H,7-9,13H2,1-2H3,(H,30,32)(H,27,28,29)/b4-3+/t16-/m0/s1
- InChI Key
- ULXXDDBFHOBEHA-CWDCEQMOSA-N
- Canonical SMILES
- CN(C)C\C=C\C(=O)NC1=C(O[C@H]2CCOC2)C=C2N=CN=C(NC3=CC(Cl)=C(F)C=C3)C2=C1
- Reference
- DrugBank
Covalent Inhibition
- Warhead
- Micheal Acceptor
- Target
-
Epidermal growth factor receptor [ UniProt: P00533 ]
- Site
- CYS-797
- Inhibition Mechanism
-
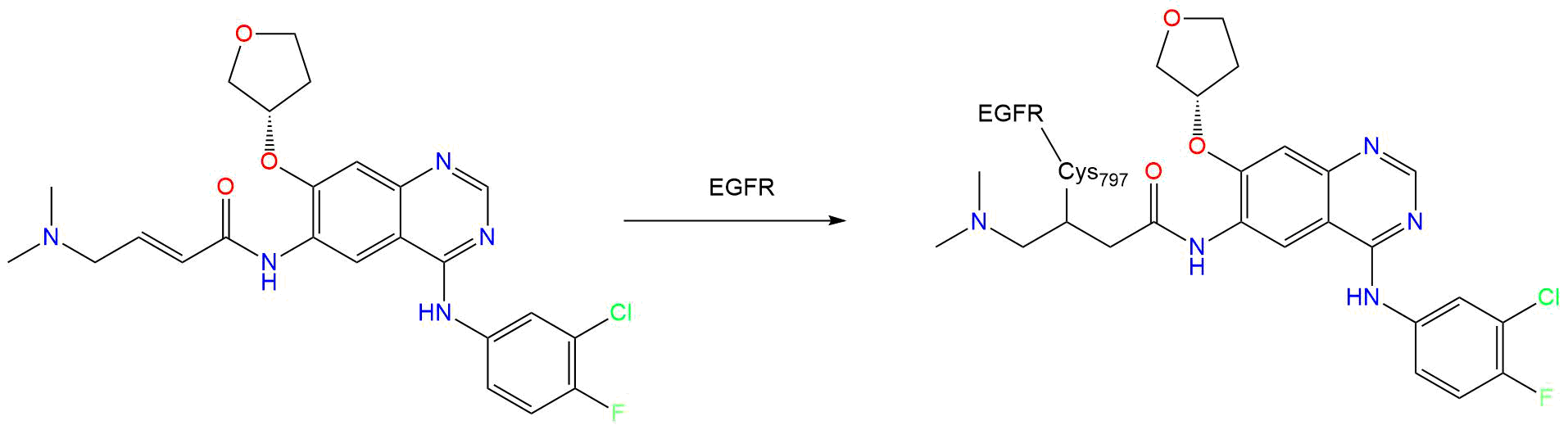
Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker
3D Structure
Calculated Properties
- logP
-
3.77
Computed by ALOGPS
- logS
-
-4.58
Computed by ALOGPS
- Heavy Atom Count
-
34
Computed by RDKit
- Ring Count
-
4
Computed by RDKit
- Hydrogen Bond Acceptor Count
-
7
Computed by RDKit
- Hydrogen Bond Donor Count
-
2
Computed by RDKit
- Rotatable Bond Count
-
8
Computed by RDKit
- Topological Polar Surface Area
-
88.61 Å2
Computed by RDKit
Similar compounds in Virtual Screening library
Similar Natural compounds
No similar natural compounds found for this drug.