Acetylsalicylic acid
Drug information
- CovInDB Drug
- DB00945
- Name
- Acetylsalicylic acid
- Molecular Formula
- C9H8O4
- Molecular Weight
- 180.04 g/mol
- Description
- Also known as Aspirin, acetylsalicylic acid (ASA) is a commonly used drug for the treatment of pain and fever due to various causes. Acetylsalicylic acid has both anti-inflammatory and antipyretic effects. This drug also inhibits platelet aggregation and is used in the prevention of blood clots stroke, and myocardial infarction (MI) and is available in many doses and forms, including chewable tablets, suppositories, extended release formulations, and others .
Acetylsalicylic acid is a very common cause of accidental poisoning in young children. It should be kept out of reach from young children, toddlers, and infants . - Status
- approved, vet_approved
- Structure
-
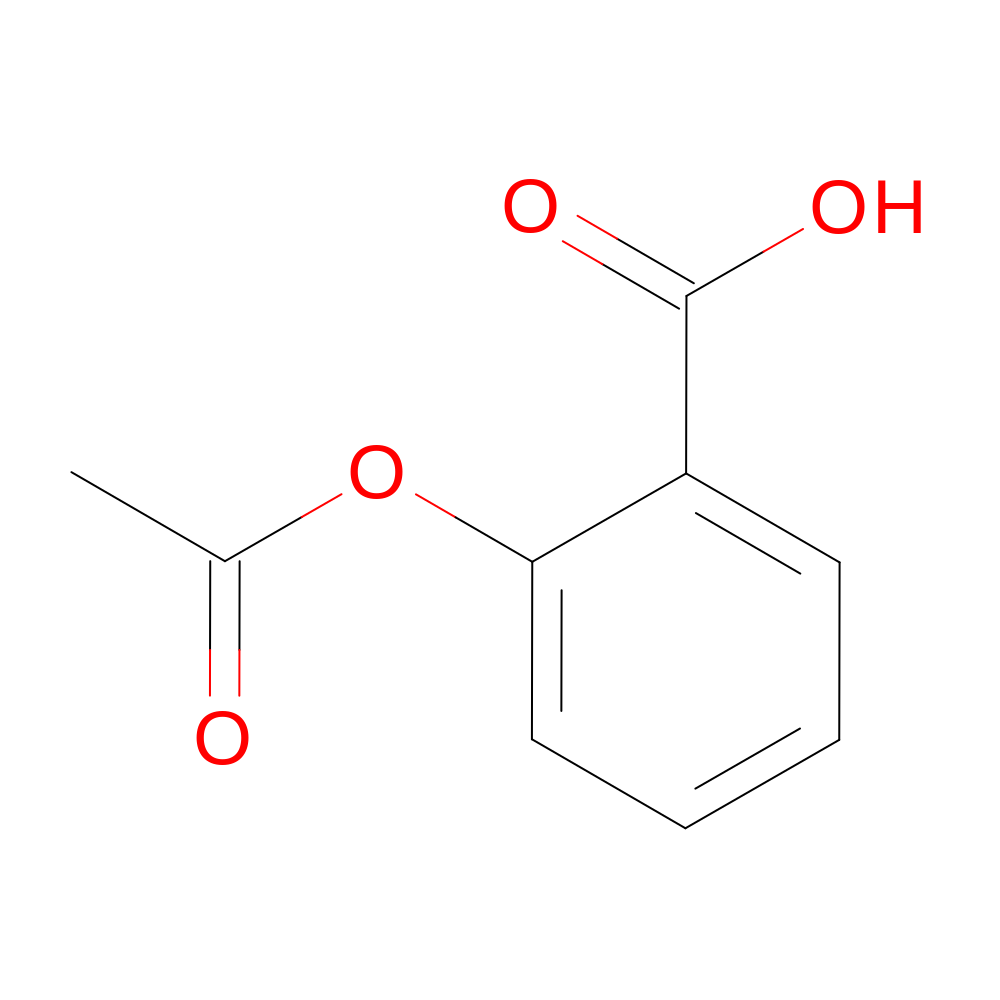
- Indication
Pain, fever, and inflammation
Acetylsalicylic acid (ASA), in the regular tablet form (immediate-release), is indicated to relieve pain, fever, and inflammation associated with many conditions, including the flu, the common cold, neck and back pain, dysmenorrhea, headache, tooth pain, sprains, fractures, myositis, neuralgia, synovitis, arthritis, bursitis, burns, and various injuries. It is also used for symptomatic pain relief after surgical and dental procedures .
The extra strength formulation of acetylsalicylic acid is also indicated for the management migraine pain with photophobia (sensitivity to light) and phonophobia (sensitivity to sound).
Other indications
ASA is also indicated for various other purposes, due to its ability to inhibit platelet aggregation. These include:
Reducing the risk of cardiovascular death in suspected cases of myocardial infarction (MI) .
Reducing the risk of a first non-fatal myocardial infarction in patients, and for reducing the risk of morbidity and mortality in cases of unstable angina and in those who have had a prior myocardial infarction .
For reducing the risk of transient ischemic attacks (TIA) and to prevent atherothrombotic cerebral infarction (in conjunction with other treatments) .
For the prevention of thromboembolism after hip replacement surgery .
For decreasing platelet to platelet adhesion following carotid endarterectomy, aiding in the prevention of transient ischemic attacks (TIA) .
Used for patients undergoing hemodialysis with a silicone rubber arteriovenous cannula inserted to prevent thrombosis at the insertion site .
Important note regarding use of the extended-release formulation
In the setting of acute myocardial infarction, or before percutaneous interventions, the extended-release form of acetylsalicylic acid should not be used. Use immediate-release formulations in scenarios requiring rapid onset of action . The extended-release form is taken to decrease the incidence of mortality and myocardial infarction (MI) for individuals diagnosed with chronic coronary artery disease (CAD), including patients with previous myocardial infarction (MI) or unstable angina or with chronic stable angina. Additionally, the extended-release form is used to decrease the risk of death and recurrent episodes of stroke in patients with a history of stroke or TIA .
- Mechanism of action
-
Acetylsalicylic acid (ASA) blocks prostaglandin synthesis. It is non-selective for COX-1 and COX-2 enzymes . Inhibition of COX-1 results in the inhibition of platelet aggregation for about 7-10 days (average platelet lifespan). The acetyl group of acetylsalicylic acid binds with a serine residue of the cyclooxygenase-1 (COX-1) enzyme, leading to irreversible inhibition. This prevents the production of pain-causing prostaglandins. This process also stops the conversion of arachidonic acid to thromboxane A2 (TXA2), which is a potent inducer of platelet aggregation . Platelet aggregation can result in clots and harmful venous and arterial thromboembolism, leading to conditions such as pulmonary embolism and stroke.
It is important to note that there is 60% homology between the protein structures of COX-1 and COX-2. ASA binds to serine 516 residue on the active site of COX-2 in the same fashion as its binding to the serine 530 residue located on the active site of COX-1. The active site of COX-2 is, however, slightly larger than the active site of COX-1, so that arachidonic acid (which later becomes prostaglandins) manages to bypass the aspirin molecule inactivating COX-2 . ASA, therefore, exerts more action on the COX-1 receptor rather than on the COX-2 receptor . A higher dose of acetylsalicylic acid is required for COX-2 inhibition . - IUPAC Name
- 2-acetoxybenzoic acid
- InChI
- InChI=1S/C9H8O4/c1-6(10)13-8-5-3-2-4-7(8)9(11)12/h2-5H,1H3,(H,11,12)
- InChI Key
- BSYNRYMUTXBXSQ-UHFFFAOYSA-N
- Canonical SMILES
- CC(=O)OC1=CC=CC=C1C(O)=O
- Reference
- DrugBank
Covalent Inhibition
- Warhead
- Ester
- Target
-
cyclooxygenase [ UniProt: P23219 ]
- Site
- SER-530
- Inhibition Mechanism
-
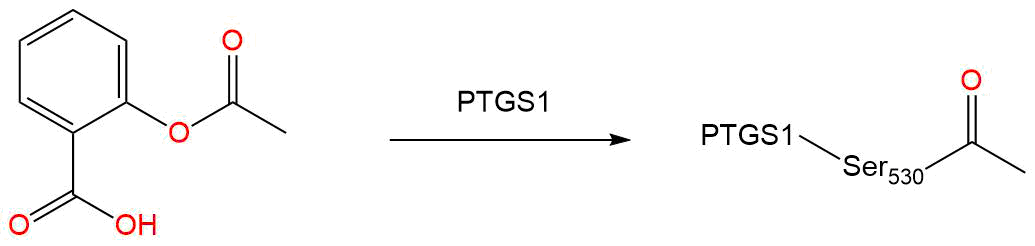
The structural basis of aspirin activity inferred from the crystal structure of inactivated prostaglandin H2 synthase
3D Structure
Calculated Properties
- logP
-
1.43
Computed by ALOGPS
- logS
-
-2.09
Computed by ALOGPS
- Heavy Atom Count
-
13
Computed by RDKit
- Ring Count
-
1
Computed by RDKit
- Hydrogen Bond Acceptor Count
-
3
Computed by RDKit
- Hydrogen Bond Donor Count
-
1
Computed by RDKit
- Rotatable Bond Count
-
2
Computed by RDKit
- Topological Polar Surface Area
-
63.6 Å2
Computed by RDKit