6YG3
Target information
- RCSB PDB
- 6YG3
- Title
- Crystal structure of MKK7 (MAP2K7) covalently bound with CPT1-70-1
- Method
- X-RAY DIFFRACTION
- Resolution
- 2.05
- Classification
- TRANSFERASE
- Organism
- Homo sapiens
- Protein
- Dual specificity mitogen-activated protein kinase kinase 7 (O14733) Looking for covalent inhibitors of this target ?
- Year
- 2020
- Publication Title
- Catalytic Domain Plasticity of MKK7 Reveals Structural Mechanisms of Allosteric Activation and Diverse Targeting Opportunities.
- Abstract
-
MKK7 (MEK7) is a key regulator of the JNK stress signaling pathway and targeting MKK7 has been proposed as a chemotherapeutic strategy. Detailed understanding of the MKK7 structure and factors that affect its activity is therefore of critical importance. Here, we present a comprehensive set of MKK7 crystal structures revealing insights into catalytic domain plasticity and the role of the N-terminal regulatory helix, conserved in all MAP2Ks, mediating kinase activation. Crystal structures harboring this regulatory helix revealed typical structural features of active kinase, providing exclusively a first model of the MAP2K active state. A small-molecule screening campaign yielded multiple scaffolds, including type II irreversible inhibitors a binding mode that has not been reported previously. We also observed an unprecedented allosteric pocket located in the N-terminal lobe for the approved drug ibrutinib. Collectively, our structural and functional data expand and provide alternative targeting strategies for this important MAP2K kinase.
- External Link
- RCSB PDB
Ligand information
- HET
- 6HF
- Chain ID
- A
- HET Number
- 510
- Molecular Formula
- C26H27N7O2
- Structure
-
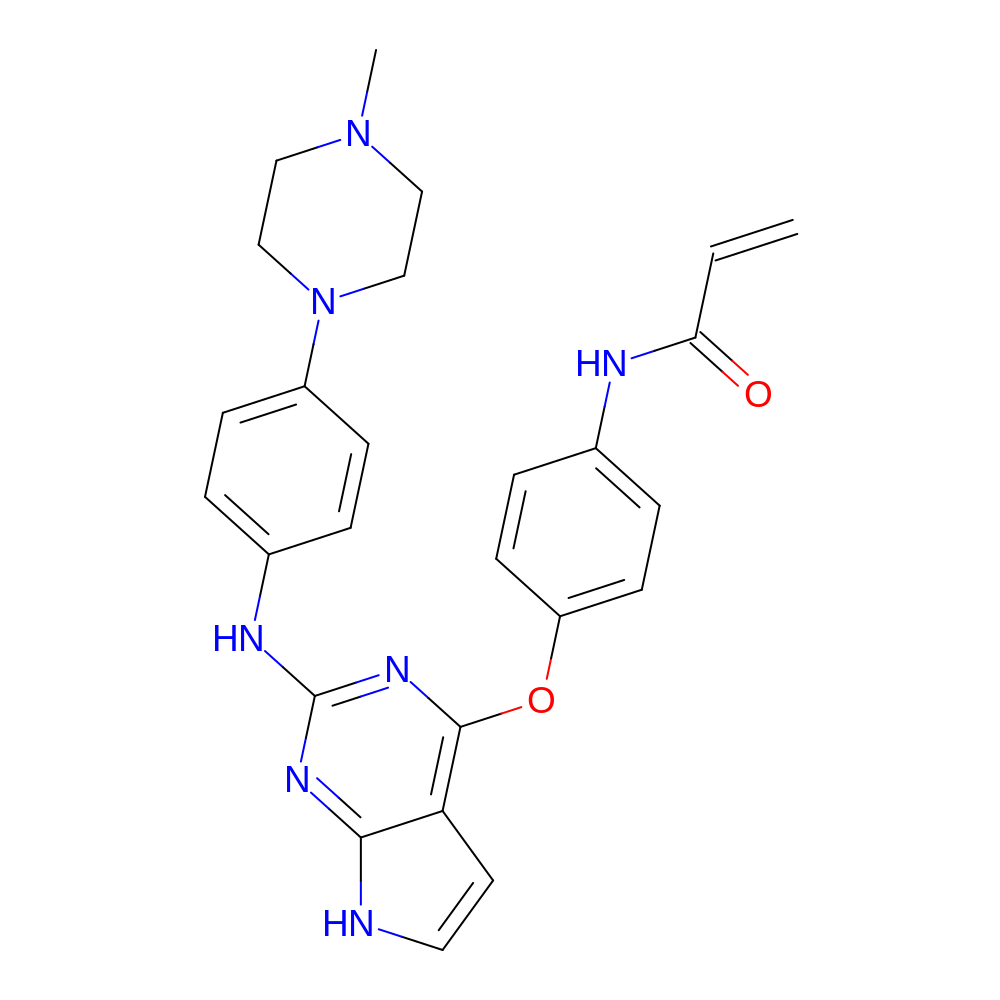
- IUPAC Name
- N-[4-[[2-[4-(4-methylpiperazin-1-yl)anilino]-7H-pyrrolo[2,3-d]pyrimidin-4-yl]oxy]phenyl]prop-2-enamide
- InChI
- InChI=1S/C26H27N7O2/c1-3-23(34)28-18-6-10-21(11-7-18)35-25-22-12-13-27-24(22)30-26(31-25)29-19-4-8-20(9-5-19)33-16-14-32(2)15-17-33/h3-13H,1,14-17H2,2H3,(H,28,34)(H2,27,29,30,31)
- InChI Key
- LPJIGUIJJBQRTQ-UHFFFAOYSA-N
- Canonical SMILES
- CN1CCN(CC1)c1ccc(Nc2nc(Oc3ccc(NC(=O)C=C)cc3)c3cc[nH]c3n2)cc1
- Bioactivity data
- CI001821
Covalent Binding
- Warhead
- Michael Acceptor
- Reaction Mechanism
- Michael Addition
- Residue
- CYS : 218
- Residue Chain
- A
- Interactions
- Pharmacophore Model