6Q7N
Target information
- RCSB PDB
- 6Q7N
- Title
- Crystal structure of BH32 alkylated with the mechanistic inhibitor 2-bromoacetophenone
- Method
- X-RAY DIFFRACTION
- Resolution
- 2.02
- Classification
- HYDROLASE
- Organism
- Pyrococcus horikoshii
- Protein
- Glyceraldehyde 3-phosphate phosphatase (O58216)
- Year
- 2018
- Publication Title
- Design and evolution of an enzyme with a non-canonical organocatalytic mechanism.
- Abstract
-
The combination of computational design and laboratory evolution is a powerful and potentially versatile strategy for the development of enzymes with new functions 1-4 . However, the limited functionality presented by the genetic code restricts the range of catalytic mechanisms that are accessible in designed active sites. Inspired by mechanistic strategies from small-molecule organocatalysis 5 , here we report the generation of a hydrolytic enzyme that uses N ?? -methylhistidine as a non-canonical catalytic nucleophile. Histidine methylation is essential for catalytic function because it prevents the formation of unreactive acyl-enzyme intermediates, which has been a long-standing challenge when using canonical nucleophiles in enzyme design 6-10 . Enzyme performance was optimized using directed evolution protocols adapted to an expanded genetic code, affording a biocatalyst capable of accelerating ester hydrolysis with greater than 9,000-fold increased efficiency over free N ?? -methylhistidine in solution. Crystallographic snapshots along the evolutionary trajectory highlight the catalytic devices that are responsible for this increase in efficiency. N ?? -methylhistidine can be considered to be a genetically encodable surrogate of the widely employed nucleophilic catalyst dimethylaminopyridine 11 , and its use will create opportunities to design and engineer enzymes for a wealth of valuable chemical transformations.
- External Link
- RCSB PDB
Ligand information
- HET
- AC0
- Chain ID
- A
- HET Number
- 301
- Molecular Formula
- C8H7BrO
- Structure
-
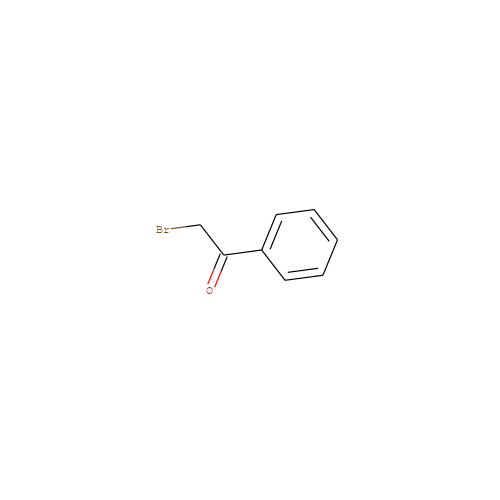
- IUPAC Name
- 2-bromo-1-phenyl-ethanone
- InChI
- InChI=1S/C8H7BrO/c9-6-8(10)7-4-2-1-3-5-7/h1-5H,6H2
- InChI Key
- LIGACIXOYTUXAW-UHFFFAOYSA-N
- Canonical SMILES
- BrCC(=O)c1ccccc1
- Bioactivity data
- CI000018
Covalent Binding
- Warhead
- Halohydrocarbon
- Reaction Mechanism
- Nucleophilic Substitution
- Residue
- HIS : 23
- Residue Chain
- A
- Interactions
- Pharmacophore Model