6N9P
Target information
- RCSB PDB
- 6N9P
- Title
- Discovery of affinity-based probes for Btk occupancy assay
- Method
- X-RAY DIFFRACTION
- Resolution
- 2.23
- Classification
- IMMUNE SYSTEM
- Organism
- Homo sapiens
- Protein
- Tyrosine-protein kinase BTK (Q06187) Looking for covalent inhibitors of this target ?
- Year
- 2018
- Publication Title
- Discovery of Affinity-Based Probes for Btk Occupancy Assays.
- Abstract
-
Bruton's tyrosine kinase (Btk) is an attractive target for the treatment of a wide array of B-cell malignancies and autoimmune diseases. Small-molecule covalent irreversible Btk inhibitors targeting Cys481 have been developed for the treatment of such diseases. In clinical trials, probe molecules are required in occupancy studies to measure the level of engagement of the protein by these covalent irreversible inhibitors. The result of this pharmacodynamic (PD) activity provides guidance for appropriate dosage selection to optimize inhibition of the drug target and correlation of target inhibition with disease treatment efficacy. This information is crucial for successful evaluation of drug candidates in clinical trials. Based on the pyridine carboxamide scaffold of a novel solvent-accessible pocket (SAP) series of covalent irreversible Btk inhibitors, we successfully developed a potent and selective affinity-based biotinylated probe 12 (2-[(4-{4-[5-(1-{5-[(3aS,4S,6aR)-2-oxo-hexahydro-1H-thieno[3,4-d]imidazol-4-yl]pentanamido}-3,6,9,12-tetraoxapentadecan-15-amido)pentanoyl]piperazine-1-carbonyl}phenyl)amino]-6-[1-(prop-2-enoyl)piperidin-4-yl]pyridine-3-carboxamide). Compound 12 has been used in Btk occupancy assays for preclinical studies to determine the therapeutic efficacy of Btk inhibition in two mouse lupus models driven by TLR7 activation and type?I interferon.
- External Link
- RCSB PDB
Ligand information
- HET
- KHD
- Chain ID
- A
- HET Number
- 701
- Molecular Formula
- C26H21N3O3
- Structure
-
- IUPAC Name
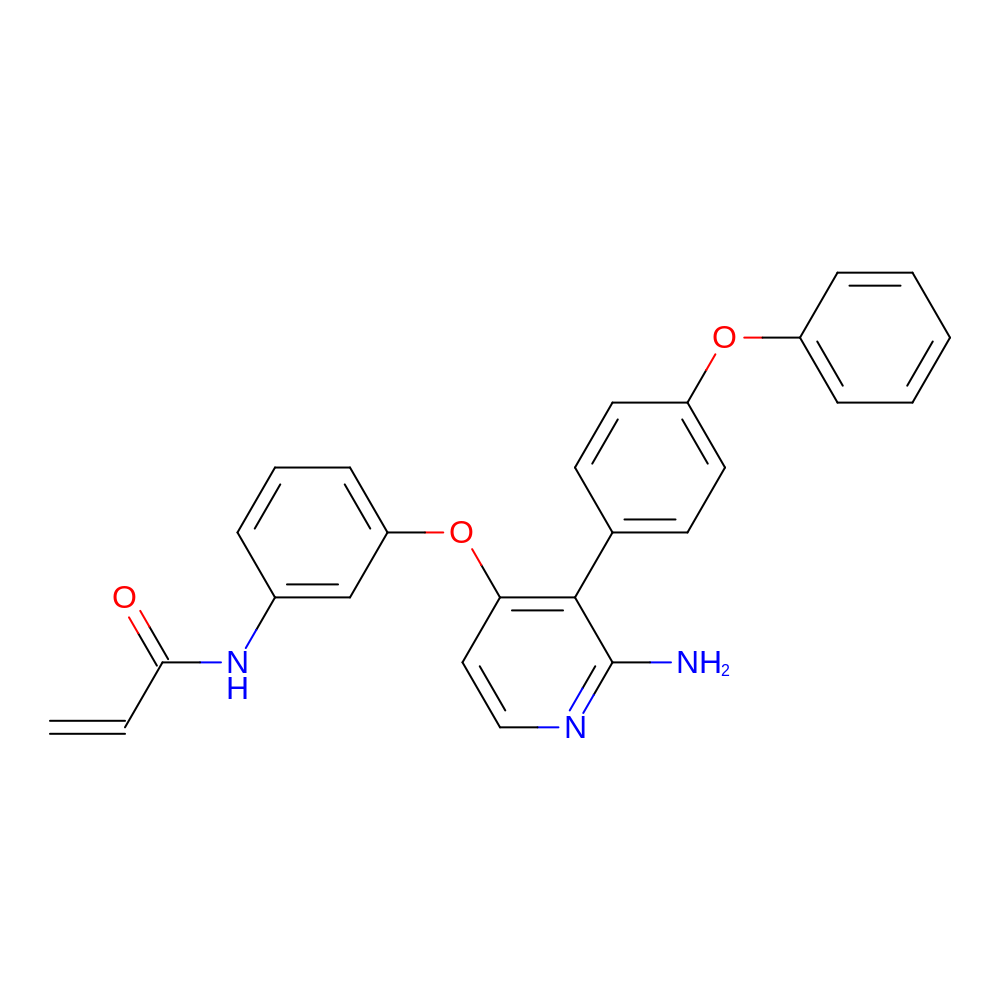
- N-[3-[[2-amino-3-(4-phenoxyphenyl)-4-pyridyl]oxy]phenyl]prop-2-enamide
- InChI
- InChI=1S/C26H21N3O3/c1-2-24(30)29-19-7-6-10-22(17-19)32-23-15-16-28-26(27)25(23)18-11-13-21(14-12-18)31-20-8-4-3-5-9-20/h2-17H,1H2,(H2,27,28)(H,29,30)
- InChI Key
- CAWFKHMTDSKZFM-UHFFFAOYSA-N
- Canonical SMILES
- Nc1nccc(Oc2cccc(NC(=O)C=C)c2)c1-c1ccc(Oc2ccccc2)cc1
- Bioactivity data
- CI004774
Covalent Binding
- Warhead
- Michael Acceptor
- Reaction Mechanism
- Michael Addition
- Residue
- CYS : 481
- Residue Chain
- A
- Interactions
- Pharmacophore Model