6MZW
Target information
- RCSB PDB
- 6MZW
- Title
- TAS-120 covalent complex with FGFR1
- Method
- X-RAY DIFFRACTION
- Resolution
- 2.2
- Classification
- TRANSFERASE/Transferase Inhibitor
- Organism
- Homo sapiens
- Protein
- Fibroblast growth factor receptor 1 (P11362) Looking for covalent inhibitors of this target ?
- Year
- 2018
- Publication Title
- TAS-120 Cancer Target Binding: Defining Reactivity and Revealing the First Fibroblast Growth Factor Receptor 1 (FGFR1) Irreversible Structure.
- Abstract
-
1-[(3S)-3-[4-Amino-3-[2-(3,5-dimethoxyphenyl)ethynyl]-1H-pyrazolo[3,4-d]pyrimidin-1-yl]-1-pyrrolidinyl]-2-propen-1-one (TAS-120) is an irreversible inhibitor of the fibroblast growth factor receptor (FGFR) family, and is currently under phase?I/II clinical trials in patients with confirmed advanced metastatic solid tumours harbouring FGFR aberrations. This inhibitor specifically targets the P-loop of the FGFR tyrosine kinase domain, forming a covalent adduct with a cysteine side chain of the protein. Our mass spectrometry experiments characterise an exceptionally fast chemical reaction in forming the covalent complex. The structural basis of this reactivity is revealed by a sequence of three X-ray crystal structures: a free ligand structure, a reversible FGFR1 structure, and the first reported irreversible FGFR1 adduct structure. We hypothesise that the most significant reactivity feature of TAS-120 is its inherent ability to undertake conformational sampling of the FGFR P-loop. In designing novel covalent FGFR inhibitors, such a phenomenon presents an attractive strategy requiring appropriate positioning of an acrylamide group similarly to that of TAS-120.
- External Link
- RCSB PDB
Ligand information
- HET
- TZ0
- Chain ID
- A
- HET Number
- 801
- Molecular Formula
- C22H22N6O3
- Structure
-
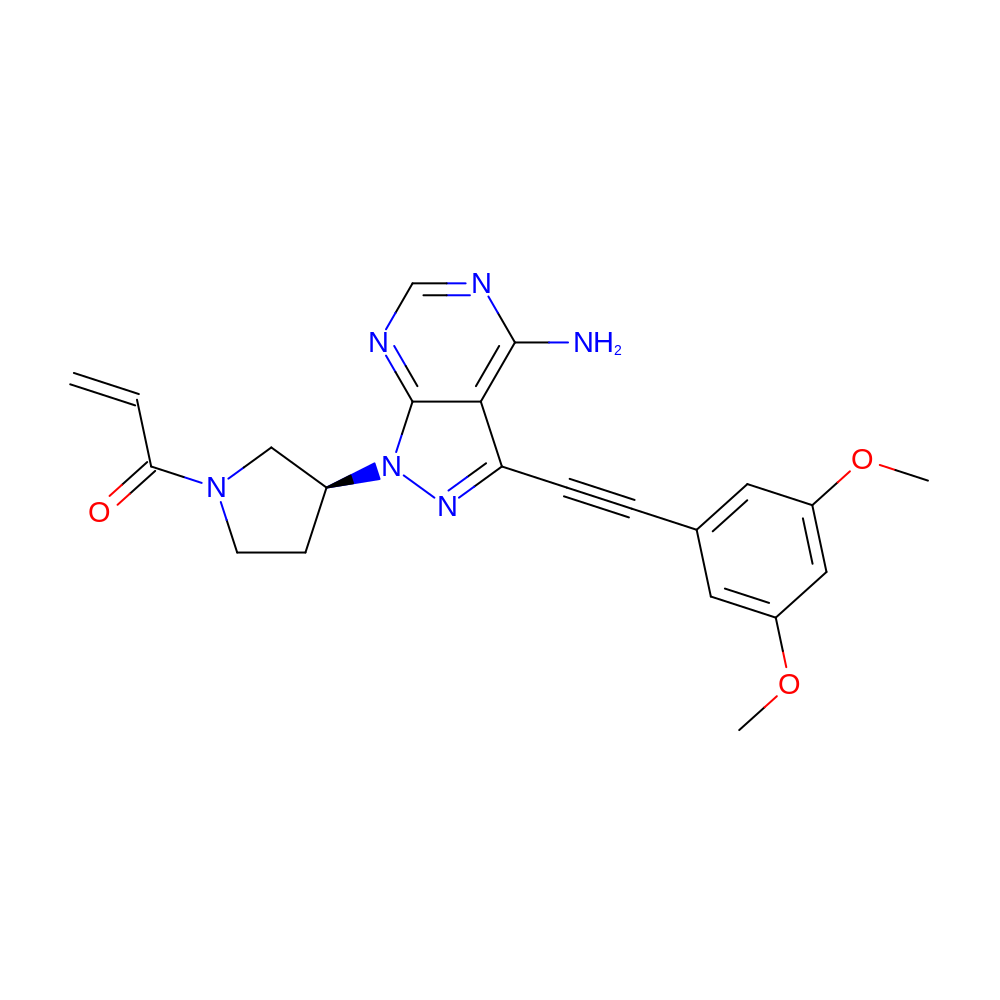
- IUPAC Name
- 1-[(3S)-3-[4-amino-3-[2-(3,5-dimethoxyphenyl)ethynyl]pyrazolo[3,4-d]pyrimidin-1-yl]pyrrolidin-1-yl]prop-2-en-1-one
- InChI
- InChI=1S/C22H22N6O3/c1-4-19(29)27-8-7-15(12-27)28-22-20(21(23)24-13-25-22)18(26-28)6-5-14-9-16(30-2)11-17(10-14)31-3/h4,9-11,13,15H,1,7-8,12H2,2-3H3,(H2,23,24,25)/t15-/m0/s1
- InChI Key
- KEIPNCCJPRMIAX-HNNXBMFYSA-N
- Canonical SMILES
- COc1cc(cc(c1)OC)C#Cc(c(c23)c(N)ncn3)nn2[C@H]4CCN(C4)C(=O)C=C
- Bioactivity data
- CI008021
Covalent Binding
- Warhead
- Michael Acceptor
- Reaction Mechanism
- Michael Addition
- Residue
- CYS : 488
- Residue Chain
- A
- Interactions
- Pharmacophore Model