6MHB
Target information
- RCSB PDB
- 6MHB
- Title
- Glutathione S-Transferase Omega 1 bound to covalent inhibitor 18
- Method
- X-RAY DIFFRACTION
- Resolution
- 2.75
- Classification
- Transferase/Transferase Inhibitor
- Organism
- Homo sapiens
- Protein
- Glutathione S-transferase omega-1 (P78417) Looking for covalent inhibitors of this target ?
- Year
- 2018
- Publication Title
- Structure-Based Design of N-(5-Phenylthiazol-2-yl)acrylamides as Novel and Potent Glutathione S-Transferase Omega 1 Inhibitors.
- Abstract
-
Using reported glutathione S-transferase omega 1 (GSTO1-1) cocrystal structures, we designed and synthesized acrylamide-containing compounds that covalently bind to Cys32 on the catalytic site. Starting from a thiazole derivative 10 (GSTO1-1 IC 50 = 0.6 ??M), compound 18 was synthesized and cocrystallized with GSTO1. Modification on the amide moiety of hit compound 10 significantly increased the GSTO1-1 inhibitory potency. We solved the cocrystal structures of new derivatives, 37 and 44, bearing an amide side chain bound to GSTO1. These new structures showed a reorientation of the phenyl thiazole core of inhibitors, 37 and 44, when compared to 18. Guided by the cocrystal structure of GSTO1:44, analogue 49 was designed, resulting in the most potent GSTO1-1 inhibitor (IC 50 = 0.22 ?? 0.02 nM) known to date. We believe that our data will form the basis for future studies of developing GSTO1-1 as a new drug target for cancer therapy.
- External Link
- RCSB PDB
Ligand information
- HET
- JR7
- Chain ID
- A
- HET Number
- 601
- Molecular Formula
- C12H9ClN2OS
- Structure
-
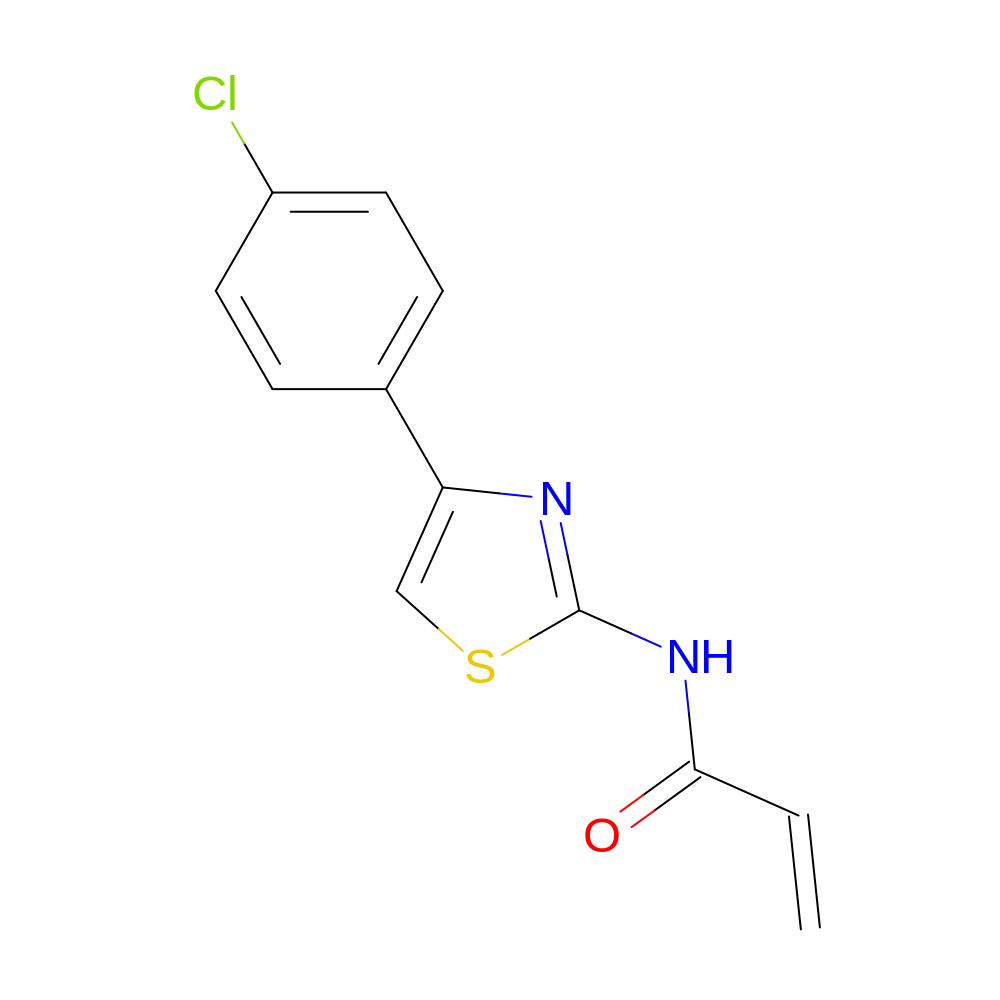
- IUPAC Name
- N-[4-(4-chlorophenyl)thiazol-2-yl]prop-2-enamide
- InChI
- InChI=1S/C12H9ClN2OS/c1-2-11(16)15-12-14-10(7-17-12)8-3-5-9(13)6-4-8/h2-7H,1H2,(H,14,15,16)
- InChI Key
- GQZUOYXKFDXAFV-UHFFFAOYSA-N
- Canonical SMILES
- C=CC(=O)Nc1nc(-c2ccc(Cl)cc2)cs1
- Bioactivity data
- CI005181
Covalent Binding
- Warhead
- Michael Acceptor
- Reaction Mechanism
- Michael Addition
- Residue
- CYS : 32
- Residue Chain
- A
- Interactions
- Pharmacophore Model