6J6M
Target information
- RCSB PDB
- 6J6M
- Title
- Co-crystal structure of BTK kinase domain with Zanubrutinib
- Method
- X-RAY DIFFRACTION
- Resolution
- 1.25
- Classification
- Transferase/Transferase inhibitor
- Organism
- Homo sapiens
- Protein
- Tyrosine-protein kinase BTK (Q06187) Looking for covalent inhibitors of this target ?
- Year
- 2019
- Publication Title
- Discovery of Zanubrutinib (BGB-3111), a Novel, Potent, and Selective Covalent Inhibitor of Bruton's Tyrosine Kinase.
- Abstract
-
Aberrant activation of Bruton's tyrosine kinase (BTK) plays an important role in pathogenesis of B-cell lymphomas, suggesting that inhibition of BTK is useful in the treatment of hematological malignancies. The discovery of a more selective on-target covalent BTK inhibitor is of high value. Herein, we disclose the discovery and preclinical characterization of a potent, selective, and irreversible BTK inhibitor as our clinical candidate by using in vitro potency, selectivity, pharmacokinetics (PK), and in vivo pharmacodynamic for prioritizing compounds. Compound BGB-3111 ( 31a , Zanubrutinib) demonstrates (i) potent activity against BTK and excellent selectivity over other TEC, EGFR and Src family kinases, (ii) desirable ADME, excellent in vivo pharmacodynamic in mice and efficacy in OCI-LY10 xenograft models.
- External Link
- RCSB PDB
Ligand information
- HET
- BA0
- Chain ID
- A
- HET Number
- 701
- Molecular Formula
- C27H29N5O3
- Structure
-
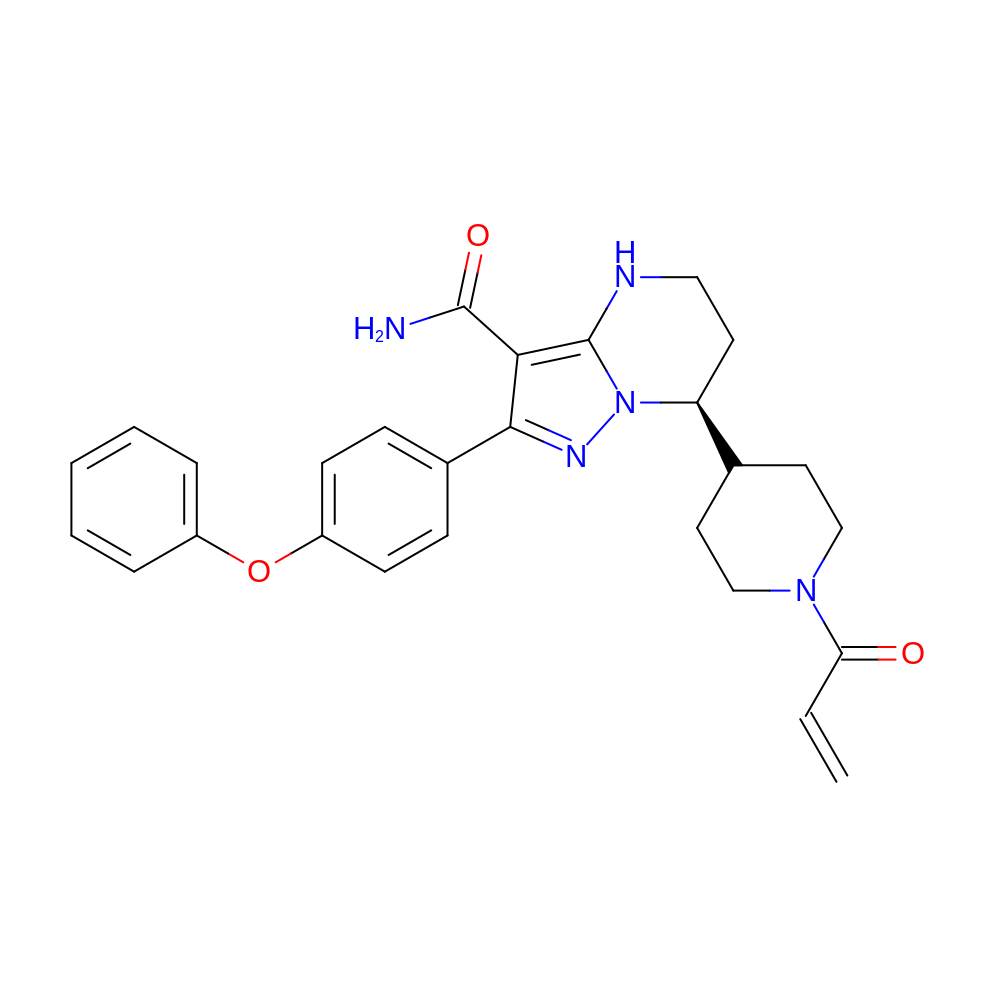
- IUPAC Name
- (7S)-2-(4-phenoxyphenyl)-7-(1-prop-2-enoyl-4-piperidyl)-4,5,6,7-tetrahydropyrazolo[1,5-a]pyrimidine-3-carboxamide
- InChI
- InChI=1S/C27H29N5O3/c1-2-23(33)31-16-13-18(14-17-31)22-12-15-29-27-24(26(28)34)25(30-32(22)27)19-8-10-21(11-9-19)35-20-6-4-3-5-7-20/h2-11,18,22,29H,1,12-17H2,(H2,28,34)/t22-/m0/s1
- InChI Key
- RNOAOAWBMHREKO-QFIPXVFZSA-N
- Canonical SMILES
- C=CC(=O)N1CCC(CC1)[C@@H]2CCNc(n23)c(C(=O)N)c(n3)-c4ccc(cc4)Oc5ccccc5
- Bioactivity data
- CI007292
Covalent Binding
- Warhead
- Michael Acceptor
- Reaction Mechanism
- Michael Addition
- Residue
- CYS : 481
- Residue Chain
- A
- Interactions
- Pharmacophore Model