6E6E
Target information
- RCSB PDB
- 6E6E
- Title
- DGY-06-116, a novel and selective covalent inhibitor of SRC kinase
- Method
- X-RAY DIFFRACTION
- Resolution
- 2.15
- Classification
- TRANSFERASE/TRANSFERASE inhibitor
- Organism
- Homo sapiens
- Protein
- Proto-oncogene tyrosine-protein kinase Src (P12931) Looking for covalent inhibitors of this target ?
- Year
- 2018
- Publication Title
- Structure and Characterization of a Covalent Inhibitor of Src Kinase.
- Abstract
-
Unregulated Src activity promotes malignant processes in cancer, but no Src-directed targeted therapies are used clinically, possibly because early Src inhibitors produce off-target effects leading to toxicity. Improved selective Src inhibitors may enable Src-directed therapies. Previously, we reported an irreversible Src inhibitor, DGY-06-116, based on the hybridization of dasatinib and a promiscuous covalent kinase probe SM1-71. Here, we report biochemical and biophysical characterization of this compound. An x-ray co-crystal structure of DGY-06-116: Src shows a covalent interaction with the kinase p-loop and occupancy of the back hydrophobic kinase pocket, explaining its high potency, and selectivity. However, a reversible analog also shows similar potency. Kinetic analysis shows a slow inactivation rate compared to other clinically approved covalent kinase inhibitors, consistent with a need for p-loop movement prior to covalent bond formation. Overall, these results suggest that a strong reversible interaction is required to allow sufficient time for the covalent reaction to occur. Further optimization of the covalent linker may improve the kinetics of covalent bond formation.
- External Link
- RCSB PDB
Ligand information
- HET
- HVY
- Chain ID
- A
- HET Number
- 601
- Molecular Formula
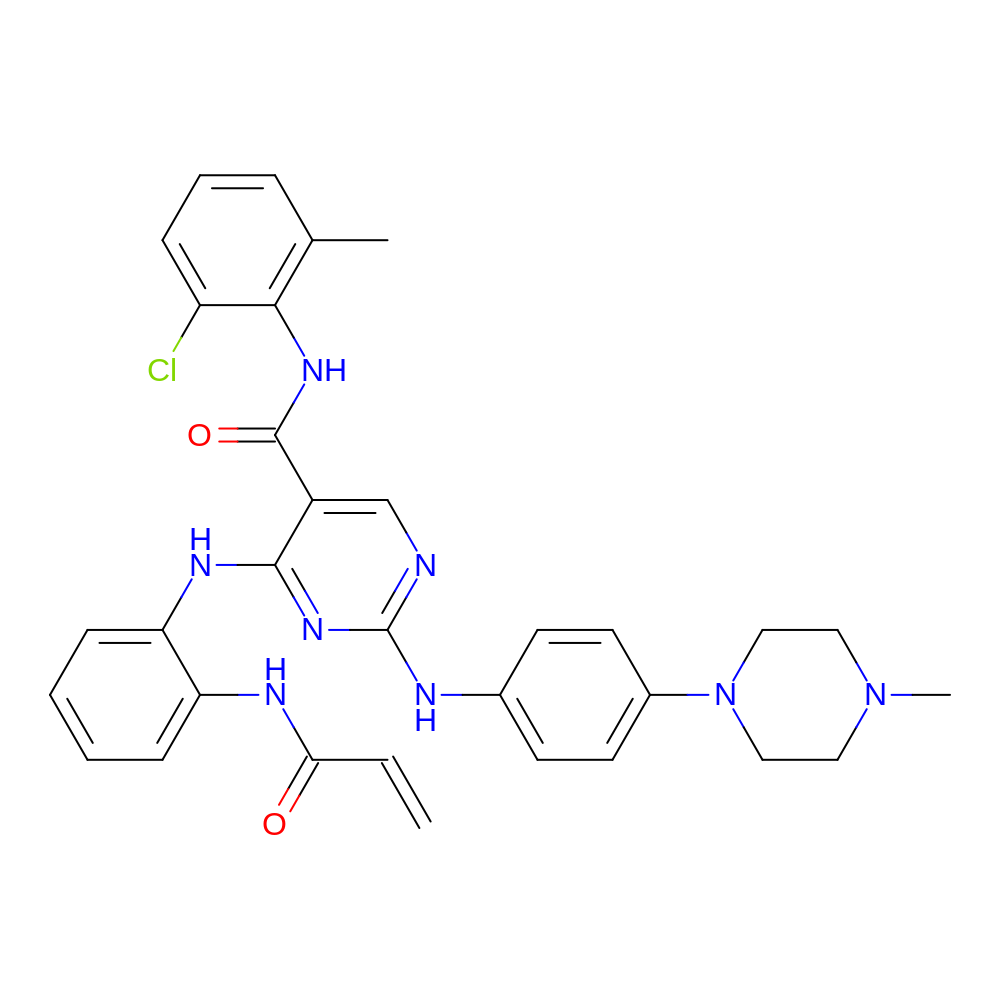
- C32H33ClN8O2
- Structure
-
- IUPAC Name
- N-(2-chloro-6-methyl-phenyl)-2-[4-(4-methylpiperazin-1-yl)anilino]-4-[2-(prop-2-enoylamino)anilino]pyrimidine-5-carboxamide
- InChI
- InChI=1S/C32H33ClN8O2/c1-4-28(42)36-26-10-5-6-11-27(26)37-30-24(31(43)38-29-21(2)8-7-9-25(29)33)20-34-32(39-30)35-22-12-14-23(15-13-22)41-18-16-40(3)17-19-41/h4-15,20H,1,16-19H2,2-3H3,(H,36,42)(H,38,43)(H2,34,35,37,39)
- InChI Key
- HLRQYOGLZWIOPA-UHFFFAOYSA-N
- Canonical SMILES
- C=CC(=O)Nc1ccccc1Nc1nc(Nc2ccc(N3CCN(C)CC3)cc2)ncc1C(=O)Nc1c(C)cccc1Cl
- Bioactivity data
- CI004533
Covalent Binding
- Warhead
- Michael Acceptor
- Reaction Mechanism
- Michael Addition
- Residue
- CYS : 280
- Residue Chain
- A
- Interactions
- Pharmacophore Model