6DI9
Target information
- RCSB PDB
- 6DI9
- Title
- CRYSTAL STRUCTURE OF BTK IN COMPLEX WITH COVALENT INHIBITOR
- Method
- X-RAY DIFFRACTION
- Resolution
- 1.25
- Classification
- TRANSFERASE/TRANSFERASE Inhibitor
- Organism
- Homo sapiens
- Protein
- Tyrosine-protein kinase BTK (Q06187) Looking for covalent inhibitors of this target ?
- Year
- 2018
- Publication Title
- Discovery of potent, highly selective covalent irreversible BTK inhibitors from a fragment hit.
- Abstract
-
Bruton's Tyrosine Kinase (BTK) is a member of the TEC kinase family that is expressed in cells of hematopoietic lineage (e.g., in B cells, macrophages, monocytes, and mast cells). Small molecule covalent irreversible BTK inhibitor targeting Cys481 within the ATP-binding pocket, for example ibrutinib, has been applied in the treatment of B-cell malignancies. Starting from a fragment hit, we discovered a novel series of potent covalent irreversible BTK inhibitors that occupy selectivity pocket of the active site of the BTK kinase domain. Guided by X-ray structures and a fragment-based drug design (FBDD) approach, we generated molecules showing comparable cellular potency to ibrutinib and higher kinome selectivity against undesirable off-targets like EGFR.
- External Link
- RCSB PDB
Ligand information
- HET
- GJJ
- Chain ID
- A
- HET Number
- 701
- Molecular Formula
- C24H30N6O3
- Structure
-
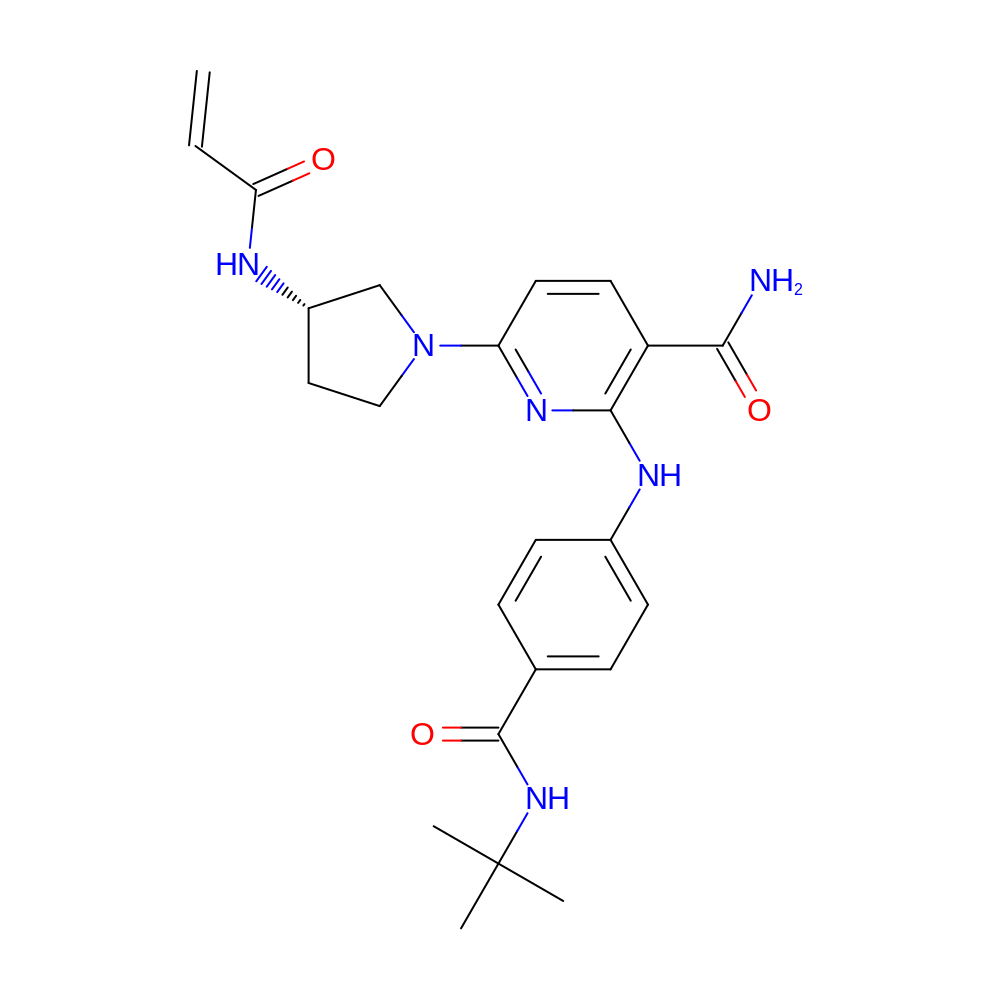
- IUPAC Name
- 2-[4-(tert-butylcarbamoyl)anilino]-6-[(3S)-3-(prop-2-enoylamino)pyrrolidin-1-yl]pyridine-3-carboxamide
- InChI
- InChI=1S/C24H30N6O3/c1-5-20(31)26-17-12-13-30(14-17)19-11-10-18(21(25)32)22(28-19)27-16-8-6-15(7-9-16)23(33)29-24(2,3)4/h5-11,17H,1,12-14H2,2-4H3,(H2,25,32)(H,26,31)(H,27,28)(H,29,33)/t17-/m0/s1
- InChI Key
- AYRMUHGKVWLINC-KRWDZBQOSA-N
- Canonical SMILES
- CC(C)(C)NC(=O)c1ccc(cc1)Nc2c(C(=O)N)ccc(n2)N3CC[C@@H](C3)NC(=O)C=C
- Bioactivity data
- CI004494
Covalent Binding
- Warhead
- Michael Acceptor
- Reaction Mechanism
- Michael Addition
- Residue
- CYS : 481
- Residue Chain
- A
- Interactions
- Pharmacophore Model