6DI1
Target information
- RCSB PDB
- 6DI1
- Title
- CRYSTAL STRUCTURE OF BTK IN COMPLEX WITH COVALENT FRAGMENT LIGAND
- Method
- X-RAY DIFFRACTION
- Resolution
- 1.1
- Classification
- TRANSFERASE/TRANSFERASE inhibitor
- Organism
- Homo sapiens
- Protein
- Tyrosine-protein kinase BTK (Q06187) Looking for covalent inhibitors of this target ?
- Year
- 2018
- Publication Title
- Discovery of a novel series of pyridine and pyrimidine carboxamides as potent and selective covalent inhibitors of Btk.
- Abstract
-
Btk is an attractive target for the treatment of a range of Bcell malignancies as well as several autoimmune diseases such as murine lupus and rheumatoid arthritis. Several covalent irreversible inhibitors of Btk are currently in development including ibrutinib which was approved for treatment of B-cell malignancies. Herein, we describe our efforts using X-ray guided structure based design (SBD) to identify a novel chemical series of covalent Btk inhibitors. The resulting pyridine carboxamides were potent and selective inhibitors of Btk having excellent enzymatic and cellular inhibitory activity.
- External Link
- RCSB PDB
Ligand information
- HET
- GJD
- Chain ID
- A
- HET Number
- 701
- Molecular Formula
- C12H16N6O2
- Structure
-
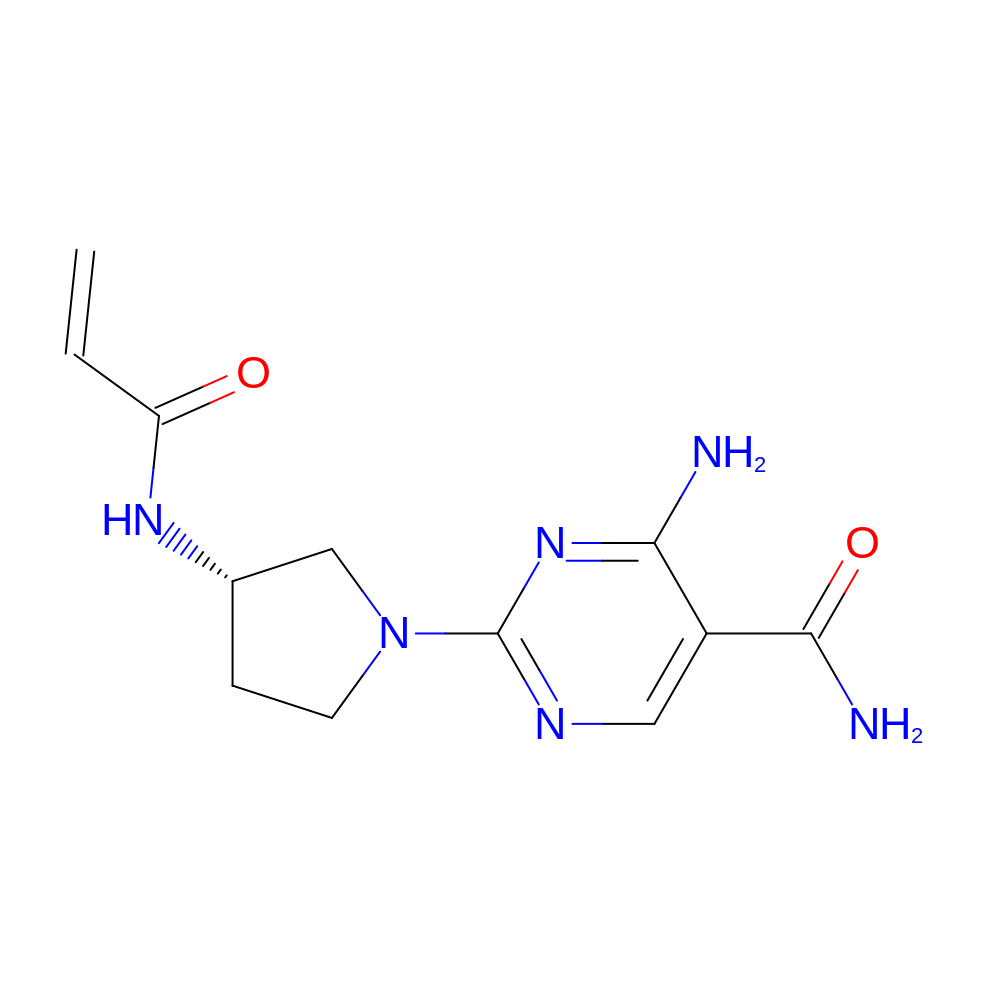
- IUPAC Name
- 4-amino-2-[(3S)-3-(prop-2-enoylamino)pyrrolidin-1-yl]pyrimidine-5-carboxamide
- InChI
- InChI=1S/C12H16N6O2/c1-2-9(19)16-7-3-4-18(6-7)12-15-5-8(11(14)20)10(13)17-12/h2,5,7H,1,3-4,6H2,(H2,14,20)(H,16,19)(H2,13,15,17)/t7-/m0/s1
- InChI Key
- XVHORVZLLWNYMP-ZETCQYMHSA-N
- Canonical SMILES
- NC(=O)c1cnc(nc1N)N2CC[C@@H](C2)NC(=O)C=C
- Bioactivity data
- CI004483
Covalent Binding
- Warhead
- Michael Acceptor
- Reaction Mechanism
- Michael Addition
- Residue
- CYS : 481
- Residue Chain
- A
- Interactions
- Pharmacophore Model