5HG5
Target information
- RCSB PDB
- 5HG5
- Title
- EGFR (L858R, T790M, V948R) in complex with N-{3-[(2-{[4-(4-methylpiperazin-1-yl)phenyl]amino}-7H-pyrrolo[2,3-d]pyrimidin-4-yl)oxy]phenyl}prop-2-enamide
- Method
- X-RAY DIFFRACTION
- Resolution
- 1.52
- Classification
- TRANSFERASE/TRANSFERASE INHIBITOR
- Organism
- Homo sapiens
- Protein
- Epidermal growth factor receptor (P00533) Looking for covalent inhibitors of this target ?
- Year
- 2016
- Publication Title
- Discovery of 1-{(3R,4R)-3-[({5-Chloro-2-[(1-methyl-1H-pyrazol-4-yl)amino]-7H-pyrrolo[2,3-d]pyrimidin-4-yl}oxy)methyl]-4-methoxypyrrolidin-1-yl}prop-2-en-1-one (PF-06459988), a Potent, WT Sparing, Irreversible Inhibitor of T790M-Containing EGFR Mutants.
- Abstract
-
First generation EGFR TKIs (gefitinib, erlotinib) provide significant clinical benefit for NSCLC cancer patients with oncogenic EGFR mutations. Ultimately, these patients' disease progresses, often driven by a second-site mutation in the EGFR kinase domain (T790M). Another liability of the first generation drugs is severe adverse events driven by inhibition of WT EGFR. As such, our goal was to develop a highly potent irreversible inhibitor with the largest selectivity ratio between the drug-resistant double mutants (L858R/T790M, Del/T790M) and WT EGFR. A unique approach to develop covalent inhibitors, optimization of reversible binding affinity, served as a cornerstone of this effort. PF-06459988 was discovered as a novel, third generation irreversible inhibitor, which demonstrates (i) high potency and specificity to the T790M-containing double mutant EGFRs, (ii) minimal intrinsic chemical reactivity of the electrophilic warhead, (iii) greatly reduced proteome reactivity relative to earlier irreversible EGFR inhibitors, and (iv) minimal activity against WT EGFR.
- External Link
- RCSB PDB
Ligand information
- HET
- 633
- Chain ID
- A
- HET Number
- 9001
- Molecular Formula
- C26H27N7O2
- Structure
-
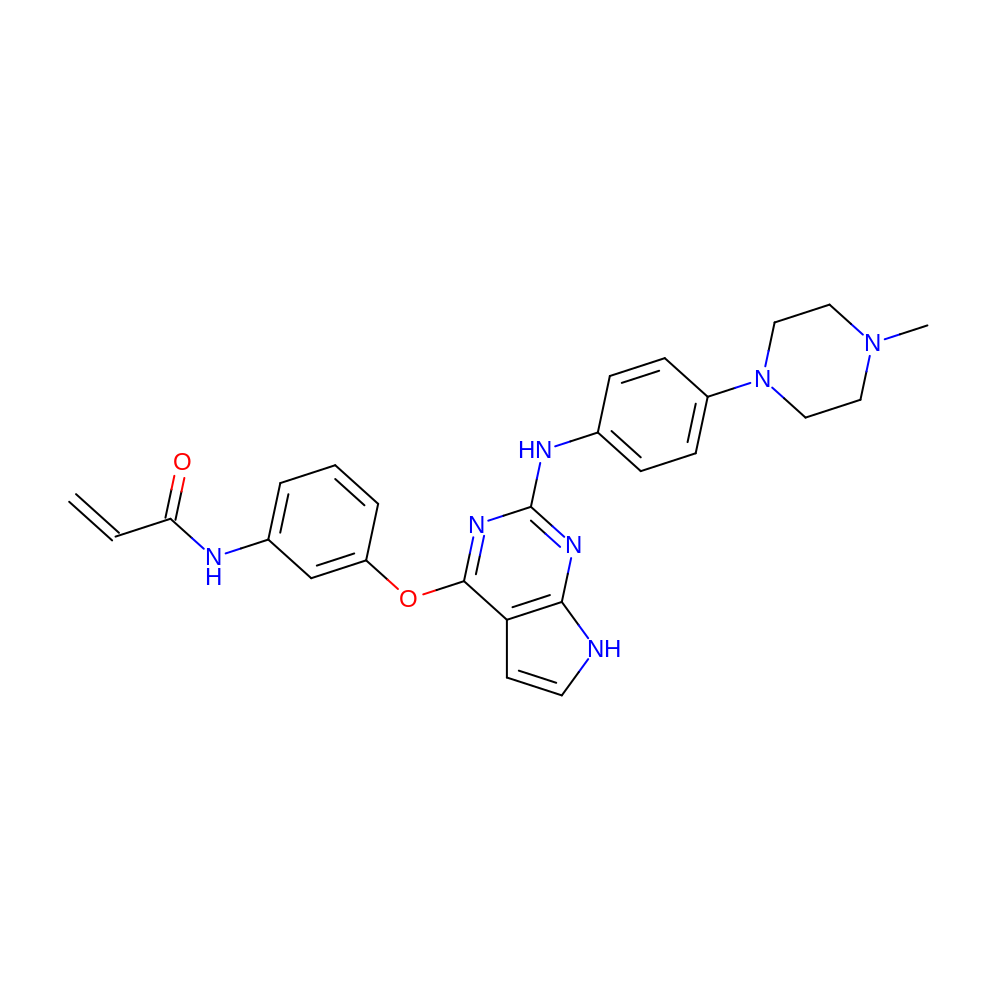
- IUPAC Name
- N-[3-[[2-[4-(4-methylpiperazin-1-yl)anilino]-7H-pyrrolo[2,3-d]pyrimidin-4-yl]oxy]phenyl]prop-2-enamide
- InChI
- InChI=1S/C26H27N7O2/c1-3-23(34)28-19-5-4-6-21(17-19)35-25-22-11-12-27-24(22)30-26(31-25)29-18-7-9-20(10-8-18)33-15-13-32(2)14-16-33/h3-12,17H,1,13-16H2,2H3,(H,28,34)(H2,27,29,30,31)
- InChI Key
- HAJRCWPFNCVVCY-UHFFFAOYSA-N
- Canonical SMILES
- C=CC(=O)Nc1cccc(Oc2nc(Nc3ccc(N4CCN(C)CC4)cc3)nc3[nH]ccc23)c1
- Bioactivity data
- CI001435
Covalent Binding
- Warhead
- Michael Acceptor
- Reaction Mechanism
- Michael Addition
- Residue
- CYS : 797
- Residue Chain
- A
- Interactions
- Pharmacophore Model