5GNK
Target information
- RCSB PDB
- 5GNK
- Title
- Crystal structure of EGFR 696-988 T790M in complex with LXX-6-34
- Method
- X-RAY DIFFRACTION
- Resolution
- 1.8
- Classification
- TRANSFERASE
- Organism
- Homo sapiens
- Protein
- Epidermal growth factor receptor (P00533) Looking for covalent inhibitors of this target ?
- Year
- 2016
- Publication Title
- Discovery of (R)-1-(3-(4-Amino-3-(3-chloro-4-(pyridin-2-ylmethoxy)phenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl)piperidin-1-yl)prop-2-en-1-one (CHMFL-EGFR-202) as a Novel Irreversible EGFR Mutant Kinase Inhibitor with a Distinct Binding Mode.
- Abstract
-
On the basis of Ibrutinib's core pharmacophore, which was moderately active to EGFR T790M mutant, we discovered novel epidermal growth factor receptor (EGFR) inhibitor compound 19 (CHMFL-EGFR-202), which potently inhibited EGFR primary mutants (L858R, del19) and drug-resistant mutant L858R/T790M. Compound 19 displayed a good selectivity profile among 468 kinases/mutants tested in the KINOMEscan assay (S score (1) = 0.02). In particular, it did not exhibit apparent activities against INSR and IGF1R kinases. The X-ray crystal structure revealed that this class of inhibitors formed a covalent bond with Cys797 in a distinct 'DFG-in-C-helix-out' inactive EGFR conformation. Compound 19 displayed strong antiproliferative effects against EGFR mutant-driven nonsmall cell lung cancer (NSCLC) cell lines such as H1975, PC9, HCC827, and H3255 but not the wild-type EGFR expressing cells. In the H1975 and PC9 cell-inoculated xenograft mouse models, compound 19 exhibited dose-dependent tumor growth suppression efficacy without obvious toxicity. Compound 19 might be a potential drug candidate for EGFR mutant-driven NSCLC.
- External Link
- RCSB PDB
Ligand information
- HET
- 80U
- Chain ID
- A
- HET Number
- 1001
- Molecular Formula
- C24H25ClN8O2
- Structure
-
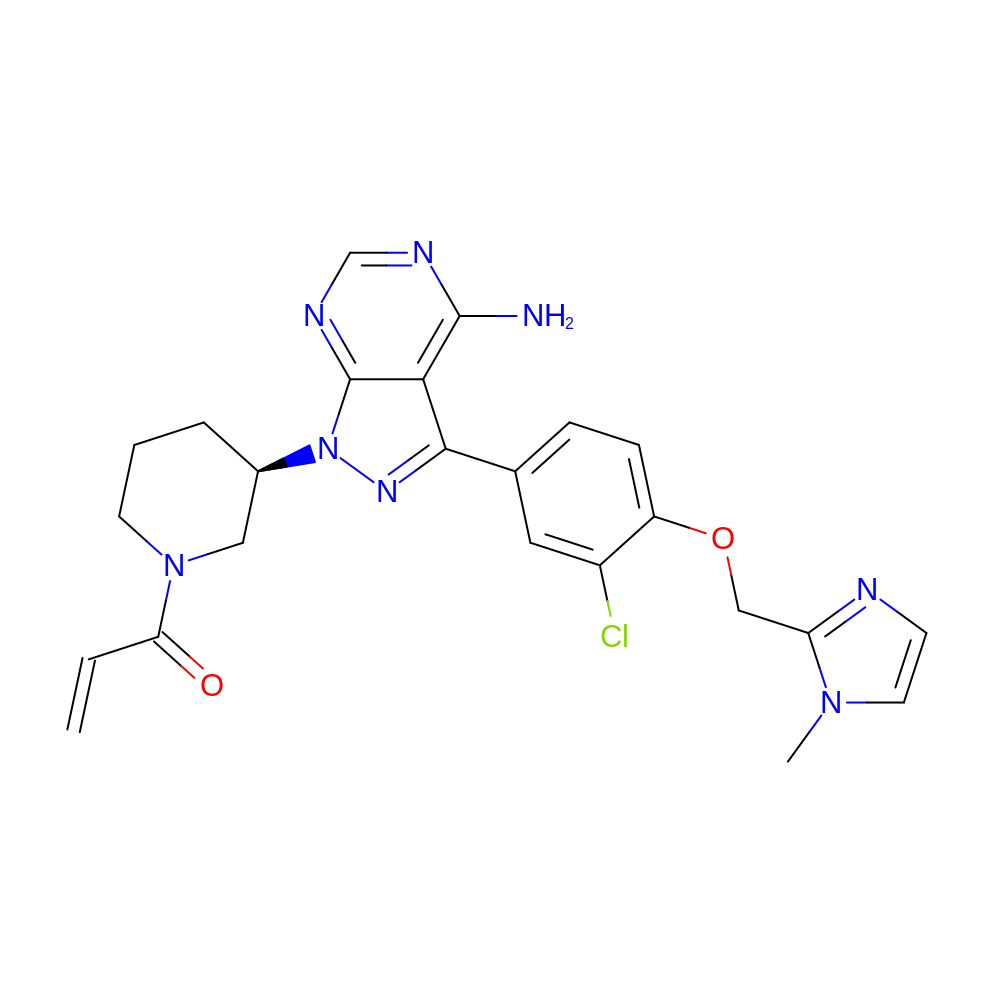
- IUPAC Name
- 1-[(3R)-3-[4-amino-3-[3-chloro-4-[(1-methylimidazol-2-yl)methoxy]phenyl]pyrazolo[3,4-d]pyrimidin-1-yl]-1-piperidyl]prop-2-en-1-one
- InChI
- InChI=1S/C24H25ClN8O2/c1-3-20(34)32-9-4-5-16(12-32)33-24-21(23(26)28-14-29-24)22(30-33)15-6-7-18(17(25)11-15)35-13-19-27-8-10-31(19)2/h3,6-8,10-11,14,16H,1,4-5,9,12-13H2,2H3,(H2,26,28,29)/t16-/m1/s1
- InChI Key
- IDPFQFBOJCHECD-MRXNPFEDSA-N
- Canonical SMILES
- Cn1ccnc1COc2ccc(cc2Cl)-c(c(c34)c(N)ncn4)nn3[C@@H]5CCCN(C5)C(=O)C=C
- Bioactivity data
- CI003725
Covalent Binding
- Warhead
- Michael Acceptor
- Reaction Mechanism
- Michael Addition
- Residue
- CYS : 797
- Residue Chain
- A
- Interactions
- Pharmacophore Model