4G5P
Target information
- RCSB PDB
- 4G5P
- Title
- Crystal structure of EGFR kinase T790M in complex with BIBW2992
- Method
- X-RAY DIFFRACTION
- Resolution
- 3.17
- Classification
- TRANSFERASE/TRANSFERASE INHIBITOR
- Organism
- Homo sapiens
- Protein
- Epidermal growth factor receptor (P00533) Looking for covalent inhibitors of this target ?
- Year
- 2012
- Publication Title
- Target Binding Properties and Cellular Activity of Afatinib (BIBW 2992), an Irreversible ErbB Family Blocker.
- Abstract
-
Deregulation of the ErbB (proto-oncogene B of the avian erythroblastosis virus AEV-H strain) receptor network is well recognized as an oncogenic driver in epithelial cancers. Several targeted drugs have been developed, including antibodies and small-molecule kinase inhibitors, each of them characterized by distinct patterns of ErbB receptor interactions. Understanding the precise pharmacological properties of these compounds is important for optimal use in clinical practice. Afatinib [BIBW 2992; N-[4-[(3-chloro-4-fluorophenyl)amino]-7-[[(3S)-tetrahydro-3-furanyl]oxy]-6-quinazolinyl]-4-(dimethylamino)-2-butenamide] is an ATP-competitive anilinoquinazoline derivative harboring a reactive acrylamide group. It was designed to covalently bind and irreversibly block enzymatically active ErbB receptor family members. Here, we show by X-ray crystallography the covalent binding of afatinib to wild-type epidermal growth factor receptor (EGFR) and by mass spectrometry the covalent interaction with EGFR, EGFRL858R/T790M, human epidermal growth factor receptor 2 (HER2), and ErbB-4. Afatinib potently inhibits the enymatic activity of ErbB-4 (EC50=1 nM) and the proliferation of cancer cell lines driven by multiple ErbB receptor aberrations at concentrations below 100 nM. N-[4-[(3-chloro-4-fluorophenyl)amino]-7-[[(3S)-tetrahydro-3-furanyl]oxy]-6-quinazolinyl]-4-(dimethylamino)-2-butanamide (BI 37781), a close analog of afatinib lacking the acrylamide group and thus incapable of covalent bond formation, had similar potency on cells driven by EGFR or EGFRL858R, but less or no detectable activity on cells expressing EGFRL858R/T790M HER2 or ErbB-4. These results stress the importance of the acrylamide group and show that afatinib differs from approved ErbB targeting agents by irreversibly inhibiting the kinase activity of all ErbB family members. They provide a mechanistic rationale for the distinct pharmacological features of this compound and explain the clinical activity seen in some patients who are resistant to antibody or kinase inhibitor therapy because of secondary mutations or ErbB receptor 'reprogramming.'
- External Link
- RCSB PDB
Ligand information
- HET
- 0WN
- Chain ID
- A
- HET Number
- 1101
- Molecular Formula
- C24H25ClFN5O3
- Structure
-
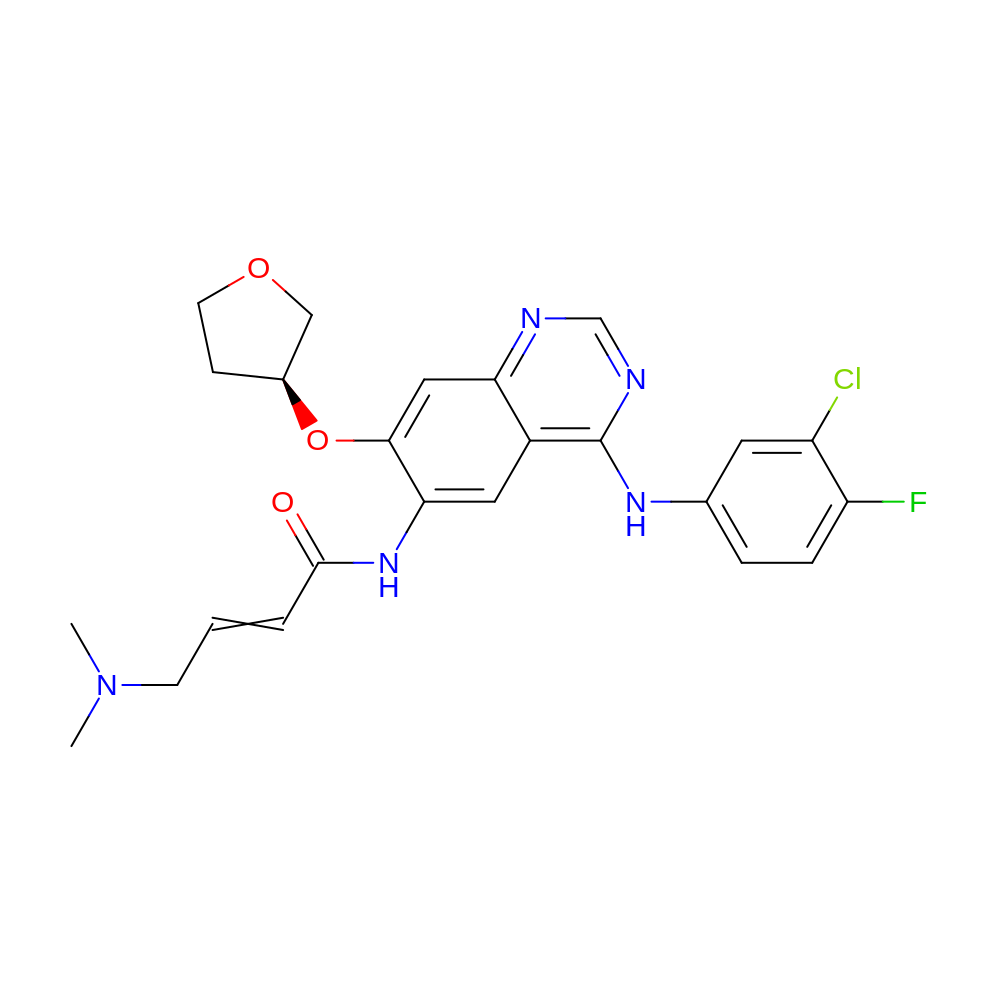
- IUPAC Name
- N-[4-(3-chloro-4-fluoro-anilino)-7-[(3S)-tetrahydrofuran-3-yl]oxy-quinazolin-6-yl]-4-(dimethylamino)but-2-enamide
- InChI
- InChI=1S/C24H25ClFN5O3/c1-31(2)8-3-4-23(32)30-21-11-17-20(12-22(21)34-16-7-9-33-13-16)27-14-28-24(17)29-15-5-6-19(26)18(25)10-15/h3-6,10-12,14,16H,7-9,13H2,1-2H3,(H,30,32)(H,27,28,29)/t16-/m0/s1
- InChI Key
- ULXXDDBFHOBEHA-INIZCTEOSA-N
- Canonical SMILES
- CN(C)CC=CC(=O)Nc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cc1O[C@H]1CCOC1
- Bioactivity data
- No bioactivity data available for this ligand.
Covalent Binding
- Warhead
- Michael Acceptor
- Reaction Mechanism
- Michael Addition
- Residue
- CYS : 797
- Residue Chain
- A
- Interactions
- Pharmacophore Model