3W2Q
Target information
- RCSB PDB
- 3W2Q
- Title
- EGFR kinase domain T790M/L858R mutant with HKI-272
- Method
- X-RAY DIFFRACTION
- Resolution
- 2.2
- Classification
- TRANSFERASE/TRANSFERASE INHIBITOR
- Organism
- Homo sapiens
- Protein
- Epidermal growth factor receptor (P00533) Looking for covalent inhibitors of this target ?
- Year
- 2012
- Publication Title
- Structure-Based Approach for the Discovery of Pyrrolo[3,2-d]pyrimidine-Based EGFR T790M/L858R Mutant Inhibitors.
- Abstract
-
The epidermal growth factor receptor (EGFR) family plays a critical role in vital cellular processes and in various cancers. Known EGFR inhibitors exhibit distinct antitumor responses against the various EGFR mutants associated with nonsmall-cell lung cancer. The L858R mutation enhances clinical sensitivity to gefitinib and erlotinib as compared with wild type and reduces the relative sensitivity to lapatinib. In contrast, the T790M mutation confers drug resistance to gefitinib and erlotinib. We determined crystal structures of the wild-type and T790M/L858R double mutant EGFR kinases with reversible and irreversible pyrrolo[3,2-d]pyrimidine inhibitors based on analogues of TAK-285 and neratinib. In these structures, M790 adopts distinct conformations to accommodate different inhibitors, whereas R858 allows conformational variations of the activation loop. These results provide structural insights for understanding the structure-activity relationships that should contribute to the development of potent inhibitors against drug-sensitive or -resistant EGFR mutations.
- External Link
- RCSB PDB
Ligand information
- HET
- HKI
- Chain ID
- A
- HET Number
- 1101
- Molecular Formula
- C30H29ClN6O3
- Structure
-
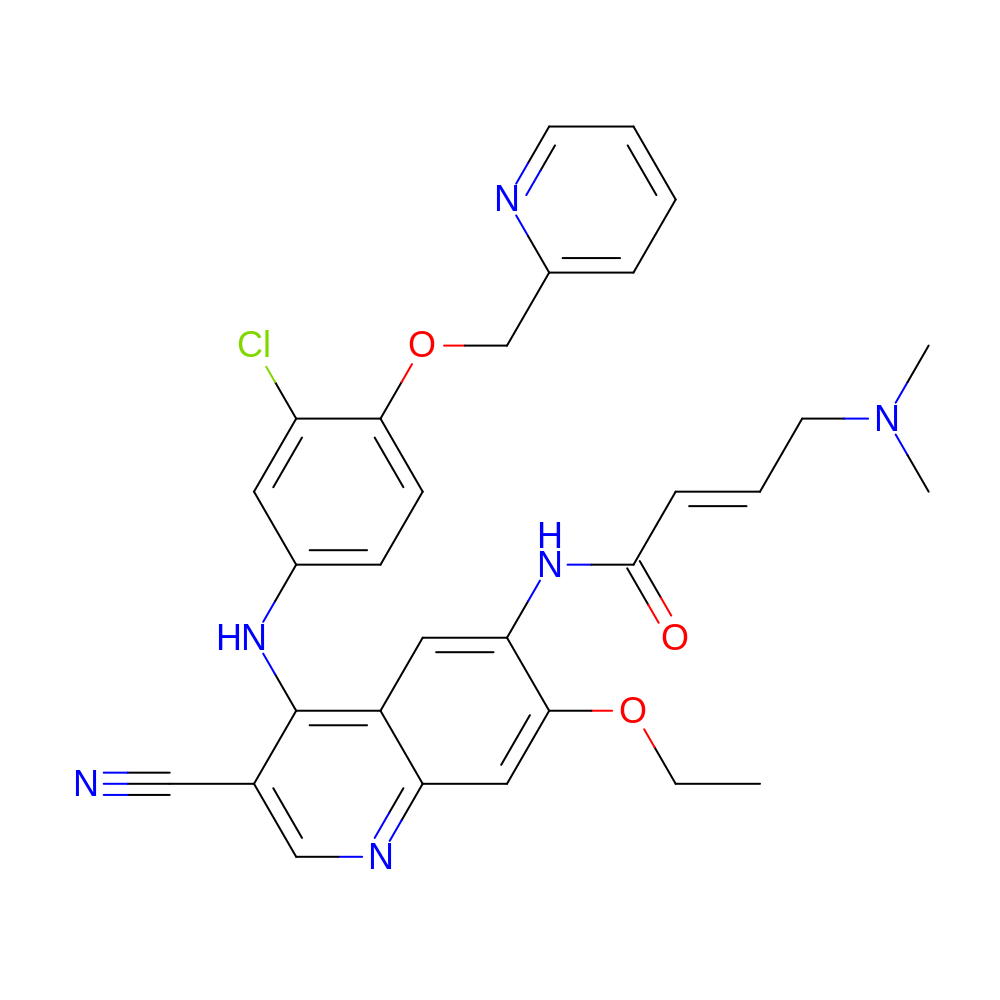
- IUPAC Name
- (E)-N-[4-[3-chloro-4-(2-pyridylmethoxy)anilino]-3-cyano-7-ethoxy-6-quinolyl]-4-(dimethylamino)but-2-enamide
- InChI
- InChI=1S/C30H29ClN6O3/c1-4-39-28-16-25-23(15-26(28)36-29(38)9-7-13-37(2)3)30(20(17-32)18-34-25)35-21-10-11-27(24(31)14-21)40-19-22-8-5-6-12-33-22/h5-12,14-16,18H,4,13,19H2,1-3H3,(H,34,35)(H,36,38)/b9-7+
- InChI Key
- JWNPDZNEKVCWMY-VQHVLOKHSA-N
- Canonical SMILES
- CCOc1cc2ncc(C#N)c(Nc3ccc(OCc4ccccn4)c(Cl)c3)c2cc1NC(=O)\C=C\CN(C)C
- Bioactivity data
- CI002489
Covalent Binding
- Warhead
- Michael Acceptor
- Reaction Mechanism
- Michael Addition
- Residue
- CYS : 797
- Residue Chain
- A
- Interactions
- Pharmacophore Model