2OK9
Target information
- RCSB PDB
- 2OK9
- Title
- PrTX-I-BPB
- Method
- X-RAY DIFFRACTION
- Resolution
- 2.34
- Classification
- TOXIN
- Organism
- Bothrops pirajai
- Protein
- Basic phospholipase A2 homolog piratoxin-1 (P58399)
- Year
- 2007
- Publication Title
- Crystal structure of a phospholipase A(2) homolog complexed with p-bromophenacyl bromide reveals important structural changes associated with the inhibition of myotoxic activity.
- Abstract
-
For the first time, the structure of a catalytic inactive phospholipase A(2) homolog (Lys49-PLA(2)s) complexed with p-bromophenacyl bromide (BPB) has been solved by X-ray crystallography. Lys49-PLA(2)s are among the main components of Viperidae snake venoms, causing myonecrosis and other actions despite their catalytic inactivity. BPB, a classic inhibitor of catalytic-active PLA(2)s, has been used since the 1970s because it binds specifically the His48 residue of the catalytic site. Curiously, when Lys49-PLA(2) is chemically modified by BPB, it causes a partial inhibition of the myotoxic function which is associated with the C-terminus and not with the catalytic site. The structure of PrTX-I complexed to BPB revealed unambiguously that the inhibitor binds covalently to His48, causing a distortion of the Ca(2)(+)-binding loop region and C-terminus rearrangement in one of its monomers. The comparison between the apo and BPB-complexed PrTX-I structures showed an increased symmetry between the two monomers with the formation of an interchain hydrogen bond between Tyr119 residues. PrTX-I undergoes tertiary and quaternary structural changes when complexed to BPB which could be related to reduction of myotoxicity and other toxic activities. We also proposed a novel myotoxic inhibition hypothesis integrating 'myotoxic' and 'active' sites for bothropic Lys49-PLA(2)s.
- External Link
- RCSB PDB
Ligand information
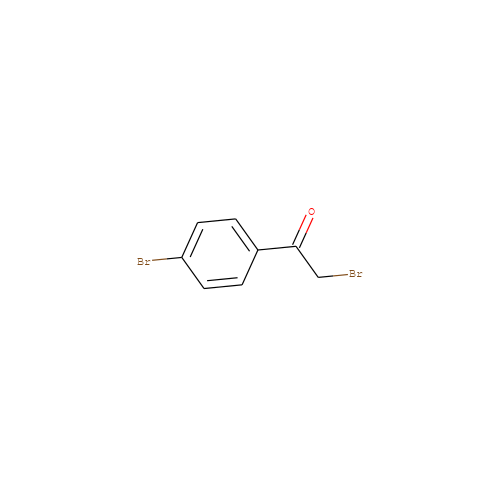
- HET
- PBP
- Chain ID
- A
- HET Number
- 248
- Molecular Formula
- C8H6Br2O
- Structure
-
- IUPAC Name
- 2-bromo-1-(4-bromophenyl)ethanone
- InChI
- InChI=1S/C8H6Br2O/c9-5-8(11)6-1-3-7(10)4-2-6/h1-4H,5H2
- InChI Key
- FKJSFKCZZIXQIP-UHFFFAOYSA-N
- Canonical SMILES
- Brc1ccc(cc1)C(=O)CBr
- Bioactivity data
- CI002419
Covalent Binding
- Warhead
- Halohydrocarbon
- Reaction Mechanism
- Nucleophilic Substitution
- Residue
- HIS : 48
- Residue Chain
- A
- Interactions
- Pharmacophore Model