2HWP
Target information
- RCSB PDB
- 2HWP
- Title
- Crystal structure of Src kinase domain in complex with covalent inhibitor PD168393
- Method
- X-RAY DIFFRACTION
- Resolution
- 2.48
- Classification
- TRANSFERASE
- Organism
- Gallus gallus
- Protein
- Proto-oncogene tyrosine-protein kinase Src (P00523) Looking for covalent inhibitors of this target ?
- Year
- 2006
- Publication Title
- Structure-guided development of affinity probes for tyrosine kinases using chemical genetics.
- Abstract
-
As key components in nearly every signal transduction pathway, protein kinases are attractive targets for the regulation of cellular signaling by small-molecule inhibitors. We report the structure-guided development of 6-acrylamido-4-anilinoquinazoline irreversible kinase inhibitors that potently and selectively target rationally designed kinases bearing two selectivity elements that are not found together in any wild-type kinase: an electrophile-targeted cysteine residue and a glycine gatekeeper residue. Cocrystal structures of two irreversible quinazoline inhibitors bound to either epidermal growth factor receptor (EGFR) or engineered c-Src show covalent inhibitor binding to the targeted cysteine (Cys797 in EGFR and Cys345 in engineered c-Src). To accommodate the new covalent bond, the quinazoline core adopts positions that are different from those seen in kinase structures with reversible quinazoline inhibitors. Based on these structures, we developed a fluorescent 6-acrylamido-4-anilinoquinazoline affinity probe to report the fraction of kinase necessary for cellular signaling, and we used these reagents to quantitate the relationship between EGFR stimulation by EGF and its downstream outputs-Akt, Erk1 and Erk2.
- External Link
- RCSB PDB
Ligand information
- HET
- DJK
- Chain ID
- A
- HET Number
- 1345
- Molecular Formula
- C17H13BrN4O
- Structure
-
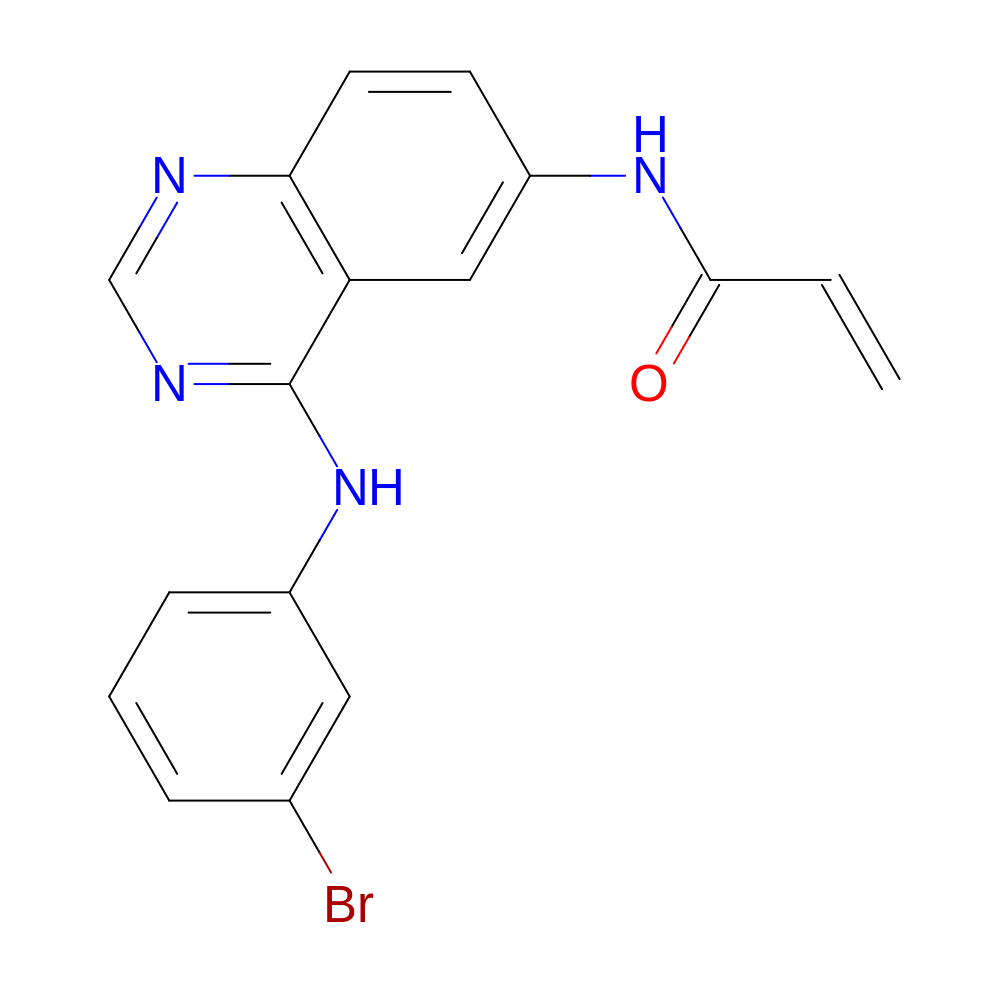
- IUPAC Name
- N-[4-(3-bromoanilino)quinazolin-6-yl]prop-2-enamide
- InChI
- InChI=1S/C17H13BrN4O/c1-2-16(23)21-13-6-7-15-14(9-13)17(20-10-19-15)22-12-5-3-4-11(18)8-12/h2-10H,1H2,(H,21,23)(H,19,20,22)
- InChI Key
- HTUBKQUPEREOGA-UHFFFAOYSA-N
- Canonical SMILES
- C=CC(=O)Nc(c1)ccc(c12)ncnc2Nc3cccc(Br)c3
- Bioactivity data
- CI002137
Covalent Binding
- Warhead
- Michael Acceptor
- Reaction Mechanism
- Michael Addition
- Residue
- CYS : 345
- Residue Chain
- A
- Interactions
- Pharmacophore Model