1BK9
Target information
- RCSB PDB
- 1BK9
- Title
- PHOSPHOLIPASE A2 MODIFIED BY PBPB
- Method
- X-RAY DIFFRACTION
- Resolution
- 2
- Classification
- HYDROLASE
- Organism
- Gloydius halys
- Protein
- Acidic phospholipase A2 (P14418)
- Year
- 1998
- Publication Title
- Structure of a snake venom phospholipase A2 modified by p-bromo-phenacyl-bromide.
- Abstract
-
The crystal structure of acidic phospholipase A2 (APLA2) from Agkistrodon halys pallas covalently modified by p-bromo-phenacyl-bromide (pBPB) was determined to a resolution of 2.0 A by an isomorphous difference Fourier method with the native APLA2 structure as an initial model and refined to a crystallographic R factor of 15.3%. The modified APLA2 structure is remarkably similar to that of the native one. Least-squares superposition of C alpha atoms of native and modified APLA2 results in a root-mean-square coordinate deviation of 0.243 A. The p-bromo-phenacyl group near the active site occupies a position similar to that in pBPB modified bovine pancreatic PLA2. The inhibitor covalently bound to the NDI atom of His48 fits well in the hydrophobic channel, forming extensive hydrophobic interactions with the surrounding residues, especially with the side chains of Phe5 and Cys45 and the main chain of Gly30. However, the inhibitor does not change the conformation of these residues except that Trp31 at the entrance of the hydrophobic channel moves slightly toward the inhibitor. Compared with native APLA2, the Ca2+-binding loop shows a little conformational change and a cation, probably Na+, occupies in the position of Ca2+. The binding of pBPB to APLA2 induce no other significant conformational changes in the enzyme molecule elsewhere.
- External Link
- RCSB PDB
Ligand information
- HET
- PBP
- Chain ID
- A
- HET Number
- 400
- Molecular Formula
- C8H6Br2O
- Structure
-
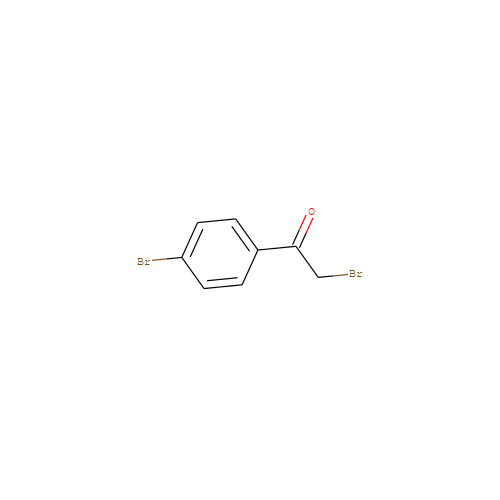
- IUPAC Name
- 2-bromo-1-(4-bromophenyl)ethanone
- InChI
- InChI=1S/C8H6Br2O/c9-5-8(11)6-1-3-7(10)4-2-6/h1-4H,5H2
- InChI Key
- FKJSFKCZZIXQIP-UHFFFAOYSA-N
- Canonical SMILES
- Brc1ccc(cc1)C(=O)CBr
- Bioactivity data
- CI002419
Covalent Binding
- Warhead
- Halohydrocarbon
- Reaction Mechanism
- Nucleophilic Substitution
- Residue
- HIS : 48
- Residue Chain
- A
- Interactions
- Pharmacophore Model